TRAPping proteins that work together inside living cells
DNA might be the blueprint for living things, but proteins are the builders. Researchers trying to understand how and which proteins work together have developed a new crosslinking tool that is small and unobtrusive enough to use in live cells. Using the new tool, the scientists have discovered new details about a well-studied complex of proteins known as RNA polymerase. The results suggest the method might uncover collaborations between proteins that are too brief for other techniques to pinpoint.
"Conventional methods used to find interacting proteins have limitations that we are trying to circumvent," said biochemist Uljana Mayer of the Department of Energy's Pacific Northwest National Laboratory. "They also create conditions that are different from those inside cells, so you can't find all the interactions that proteins would normally engage in."
Proteins are the workhorses in an organism's cells. Whole fields of research are dedicated to teasing out which proteins work together to make cells function. For example, drug researchers seek chemicals that disrupt or otherwise change how proteins interact to combat diseases; environmental scientists need to understand how proteins collaborate in ecosystems to make them thrive or fail.
To learn about protein networks, scientists start with a familiar one and use it as bait to find others that work alongside it. To pin down the collaborators, researchers make physical connections between old and new proteins with chemicals called crosslinkers. The sticky crosslinkers will only connect proteins close enough to work together, the thinking goes. But most crosslinkers are too large to squeeze into living cells, are harmful to cells, or link proteins that are neighbors but not coworkers.
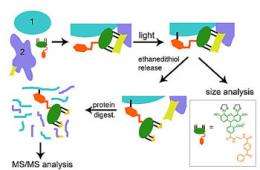
To address these issues, Mayer and her PNNL colleagues developed a crosslinking method that uses small crosslinkers whose stickiness can be carefully controlled. To find coworkers of a protein of interest, Mayer and her colleagues build a tiny molecule called a tag into the initial protein. They then add a small molecule called TRAP to the living cell, which finds and fits into the tag like two pieces in a puzzle. TRAP waves around, bumping into nearby proteins. The scientists control TRAP with a flash of light, causing it to stick to coworkers it bumps into. The researchers then identify the new "TRAPped" proteins in subsequent analyses.
To demonstrate how well this method works, Mayer and colleagues tested it out on RNA polymerase, a well-studied machine in cells. The polymerase is made up of many proteins that cooperate to translate DNA. One of the polymerase proteins has a tail that is known to touch the DNA and some helper proteins just before the polymerase starts translating. No one knew if this tail -- also known as the C-terminus of the alpha subunit -- touches anything else in the core of the RNA polymerase complex.
The team engineered a tag in the C-terminus and cultured bacteria with the tagged RNA polymerase. After adding TRAP to the cells and giving it time to find the C-terminus tag, the team shined a light on the cultures.
The team then identified the proteins marked with TRAP using instruments in EMSL, DOE's Environmental Molecular Sciences Laboratory on the PNNL campus. They found that the tagged protein, as expected, interacts with many other proteins, for example previously identified helper proteins, so-called transcription factors. But they also found it on another core protein called the beta subunit, suggesting the tail of the alpha subunit makes contact with the beta subunit as it plugs along. This interaction had never been seen before.
"No one knows what the polymerase looks like when it is running," said Uljana Mayer. "Here we see the C-terminus swings back to grab the beta subunit once the polymerase starts working."
The team report their results June 15 in the journal ChemBioChem. The tag in their unique method is made up of a "tetracysteine motif" -- two pairs of the amino acid cysteine separated by two other amino acids that doesn't interfere with the normal function of the protein of interest. TRAP includes a small "biarsenical" probe, which fluoresces so the team can find the proteins to which it has become attached. TRAP can also be easily unlinked from the tag with a simple biochemical treatment, allowing researchers to piece out the coworker from their original protein of interest.
The team also tested the method on other proteins, such as those found in young muscle cells. Mayer said they will use the method in the future to understand how environmental conditions affect how proteins work together in large networks.
Source: Pacific Northwest National Laboratory (news : web)