Plant Min protein sits tight and rescues E. coli
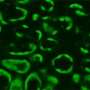
A protein vital for correct chloroplast division in plants is able to take on a similar role in bacterial cells, according to research published today in the open access journal BMC Microbiology. The Arabidopsis thaliana Min protein (AtMinD) localizes in E. coli cells' polar regions keeping cell division at its correct central location, yet unlike its E. coli homologue, AtMinD does not oscillate.
Making certain that E. coli cells divide in the centre is down to Min proteins (MinC, D and E). MinE oscillates from the middle of the cell to one pole or another, driving the MinCD complex with it. The MinCD complex prevents FtsZ polymerization at the poles but not at the mid-line of the cell, where FtsZ ring formation leads to cell division.
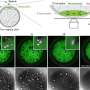
A team of Beijing-based scientists expressed the Arabidopsis MinD gene (AtMinD), in E. coli cells that lacked the bacterial genes for both MinD and MinE. Surprisingly, the minicell phenotype of this E. coli HL1 mutant (MinDE) was rescued by the plant AtMinD gene, even though the dynamic MinE protein was absent. The Arabidopsis homologue AtMinD behaved differently from its E. coli counterpart in that it did not oscillate between poles, instead taking a stand at the pointed ends (puncta) of the poles of E. coli cells. The scientists went on to show that the rescue by plant AtMinD required E. coli MinC, and that AtMinD bound EcMinC in these puncta, This is another remarkable finding because while Arabidopsis (and other plants) encode plastid homologs of bacterial MinD and MinE, MinC is either absent or has diverged beyond recognition.
"The complementation of E. coli HL1 mutant (MinDE) by AtMinD and the requirement of EcMinC for this complementation suggest that the function of MinD is conserved between bacteria and plants," says Yikun He, a member of the research team. "However, this complementation doesn't require the presence of EcMinE suggesting that AtMinD may have some characters different from that of EcMinD."
Exactly why and how the AtMinD localizes to the polar region in E. coli cells is unknown, but one possibility is a mechanism similar to that found in Bacillus subtilis. In this bacterium, MinCD proteins are localized to polar regions without oscillation and there is no MinE. Instead another protein, known as DivIVA, tethers MinCD to cell poles, preventing division at the cell ends.
Chloroplasts originated from cyanobacteria that colonised primitive plant cells, and the conservation of MinD, MinE and FtsZ genes in plants was already an indication of some conservation of function. Nonetheless it is unexpected and exciting to find that plant MinD can collaborate with bacterial MinC to convert E. coli from an oscillating to a Bacillus-type mechanism of Min action, and this finding opens new avenues for exploring Min function in both bacteria and plants.
More information: A plant MinD homologue rescues Escherichia coli HL1 mutant (Delta MinDE) in the absence of MinE, Min Zhang, Yong Hu, Jingjing Jia, Hongbo Gao and Yikun He, BMC Microbiology (in press), www.biomedcentral.com/bmcmicrobiol
Source: BioMed Central (news : web)