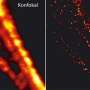
A guide to the invisible: Doubling the fluorescence microscopy resolution (w/Video)
(PhysOrg.com) -- A crucial tool in the evolution of scientific capability in bioscience, the fluorescence microscope has allowed a generation of scientists to study the properties of proteins inside cells. Yet as human capacity for discovery has zoomed to the nanoscale, fluorescence microscopy has struggled to keep up. Now, a team that includes UGA engineer Peter Kner has developed a microscope that is capable of live imaging at double the resolution of fluorescence microscopy using structured illumination.
The research was published in Nature Methods on April 26.
The laws of physics have limited the resolution of fluorescence microscopy, whereby a fluorescent marker is used to distinguish specific proteins, to about 200 nanometers. At this resolution significant detail is lost about the activity within a cell. The increased resolution by structured illumination is an engineering feat that will help scientists learn more about cell behavior and study mechanisms important for human disease.
“Our understanding of what is going on inside cells and our ability to manipulate them has advanced so much that it has become more and more important to see them at a better resolution,” said Kner, who joined UGA this spring semester. Kner built the structured illumination microscope with colleagues at the University of California, San Francisco.
This work follows on at least a decade of research building on the nearly fifty-year history of fluorescence microscopy. The technology has been a multi-disciplinary springboard of optical engineering, chemistry and biology, in which the disciplines have all contributed to visualizing fluorescent dyes attached to proteins, advancing our understanding of cellular activity. The importance of fluorescence microscopy was recently recognized with the 2008 Nobel Prize for Chemistry which was awarded for the development of the green fluorescent protein (GFP), which has played a crucial role in our identification and understanding of proteins.
“What we’ve done is develop a much faster system that allows you to look at live cells expressing GFP, which is a very powerful tool for labeling inside the cell,” Kner explained.
“It would be difficult to overstate the importance of bio-imaging to ongoing research in human health,” said Dale Threadgill, director of the UGA Faculty of Engineering. “The ability to shine a light on the leading-edge of scientific discovery will define the route to entirely new regimens of health management at the intersections of science and engineering, and Dr. Kner has joined a distinguished cadre at UGA to continue working at that interface,” Threadgill added.
Provided by University of Georgia