Scientists show why anti-HIV antibodies are ineffective at blocking infection
Some 25 years after the AIDS epidemic spawned a worldwide search for an effective vaccine against the human immunodeficiency virus (HIV), progress in the field seems to have effectively become stalled. The reason? According to new findings from a team of researchers from the California Institute of Technology (Caltech), it's at least partly due to the fact that our body's natural HIV antibodies simply don't have a long enough reach to effectively neutralize the viruses they are meant to target.
Their findings were published last week in the online early edition of the Proceedings of the National Academy of Sciences (PNAS).
"This study helps to clarify the obstacles that antibodies face in blocking infection," says Pamela Bjorkman, the Max Delbrück Professor of Biology at Caltech and a Howard Hughes Medical Institute Investigator, "and will hopefully shed more light on why developing an effective vaccine for HIV has proven so elusive."
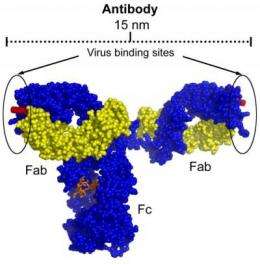
Y-shaped antibodies are best at neutralizing viruses--i.e., blocking their entry into cells and preventing infection--when both arms of the Y are able to reach out and bind to their target proteins at more or less the same time. In the case of HIV, antibodies that can block infection target the proteins that stud the surface of the virus, which stick out like spikes from the viral membrane. But an antibody can only bind to two spikes at the same time if those spikes fall within its span--the distance the antibody's structure allows it to stretch its two arms.
"When both arms of an antibody are able to bind to a virus at the same time," says Joshua Klein, a Caltech graduate student in biochemistry and molecular biophysics and the PNAS paper's first author, "there can be a hundred- to thousandfold increase in the strength of the interaction, which can sometimes translate into an equally dramatic increase in its ability to neutralize a virus. Having antibodies with two arms is nature's way of ensuring a strong binding interaction."
As it turns out, this sort of double-armed binding is easier said than done--at least in the case of HIV.
In their PNAS paper, Bjorkman and Klein looked at the neutralization capabilities of two different monoclonal antibodies isolated from HIV-infected individuals. One, called b12, binds a protein known as gp120, which forms the upper portion of an HIV's protein spike. The other, 4E10, binds to gp41, which is found on a lower portion of the spike known as the stalk.
The researchers broke each of the antibodies down into their component parts and compared their abilities to bind and neutralize the virus. They found, as expected, that one-armed versions of the b12 antibody were less effective at neutralizing HIV than two-armed versions. When they looked at the 4E10 antibody, by comparison, they found that having two arms conferred almost no advantage over having only one arm. In addition, they found that larger versions of 4E10 were less effective than smaller ones. These results highlight potential obstacles that vaccines designed to elicit antibodies similar to 4E10 might face.
But b12 has its own obstacles to overcome as well. In fact, when the researchers looked more closely at their data, they realized that the benefits of having two arms--even for b12--were much smaller than those seen for antibodies against viruses like influenza. In other words, the body's natural anti-HIV antibodies are much less effective at neutralizing HIV than they should be.
But why?
"The story really starts to get interesting when we think about what the human immunodeficiency virus actually looks like," says Klein. Whereas a single influenza virus's surface is studded with approximately 450 spikes, he explains, the similarly sized HIV may have fewer than 15 spikes.
With spikes so few and far between, finding two that both fall within the reach of a b12 or 4E10 antibody--the spans of which generally measure between 12 and 15 nanometers--becomes much more of a challenge.
"HIV may have evolved a way to escape one of the main strategies our immune system uses to defeat infections," says Klein. "Based on these data, it seems that the virus is circumventing the bivalent effect that is so key to the potency of antibodies."
"I consider this a very important paper because it changes the focus of the discussion about why anti-HIV antibodies are so poor," adds virologist David Baltimore, the Robert Andrews Millikan Professor of Biology and a Nobel Prize winner. "It brings attention to a long-recognized but often forgotten aspect of antibody attack--that they attack with two heads. What this paper shows is that anti-HIV antibodies are restricted to using one head at a time and that makes them bind much less well. Responding to this newly recognized challenge will be difficult because it identifies an intrinsic limitation on the effectiveness of almost any natural anti-HIV antibodies."
In addition to Bjorkman and Klein, the authors on the PNAS paper, "Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10," are Caltech research technicians Priyanthi Gnanapragasam, Rachel Galimidi, and Christopher Foglesong, and senior research specialist Anthony West, Jr.
The work described in the paper was supported by a Bill and Melinda Gates Foundation Grant through the Grand Challenges in Global Health Initiative and the Collaboration for AIDS Vaccine Discovery.
Source: California Institute of Technology (news : web)















