Researchers discover new fat-fighting pathway
Researchers at Albert Einstein College of Medicine of Yeshiva University have discovered a process that controls the amount of fat that cells store for use as a back-up energy source. Disruption of this process allows cellular fat to accumulate — a key factor in age-related metabolic diseases such as obesity and type 2 diabetes. The study is published today in the online version of Nature.
Discovery of this previously unknown fat-fighting pathway could lead to novel drugs for the treatment of metabolic syndrome (characterized by obesity, blood lipid disorders, and insulin resistance) and for a common liver disease known as "fatty liver" or steatohepatitis. Nonalcoholic steatohepatitis (NASH) is a common, often "silent" liver disease. Although NASH resembles alcoholic liver disease, it occurs in people who drink little or no alcohol. NASH affects 2 to 5 percent of Americans, according to the National Institute of Diabetes and Digestive and Kidney Diseases.
All cells store lipids, a type of fat, in the form of small droplets that can be broken down for energy when needed. In situations of excessive food intake or in certain diseases such as diabetes or obesity, these lipid droplets become so large that they interfere with normal cell function.
"In this study, we found that the amount of fat stored in these intracellular lipid droplets is controlled through autophagy, a process until now thought to help primarily in digesting and recycling damaged cellular structures," says Mark Czaja, M.D., professor of medicine at Einstein whose team worked collaboratively on the research with the laboratory of Ana Maria Cuervo, M.D., Ph.D., associate professor of developmental & molecular biology, medicine, and anatomy & structural biology at Einstein.
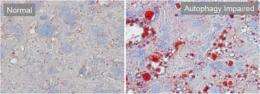
Autophagy, or "self-eating," is carried out by lysosomes, which function as the cell's recycling center. In studies of liver cells in culture and in live animals, Dr. Czaja and his colleagues discovered that lysosomes do something never before observed: continuously remove portions of lipid droplets and process them for energy production.
"When food is scarce, autophagy becomes a main source of energy for the cells and this process of digesting lipid droplets is accelerated," says Dr. Cuervo. "If autophagy slows down, as occurs in aging, the lipid droplets stored in cells keep growing and eventually become so big that they can no longer be degraded."
This slowdown in fat control appears to trigger a vicious cycle in which the enlarging fat droplets impair autophagy, allowing even more fat to accumulate, and so on, which could eventually contribute to diseases such as diabetes. The researchers noted that therapies aimed at helping autophagy operate more efficiently might prevent disease by keeping fat droplets under control.
Drs. Cuervo and Czaja's paper, "Autophagy regulates lipid metabolism" is published in the April 1 online version of Nature. Their co-authors at Einstein include Rajat Singh and Susmita Kaushik (primary co-authors), Yongjun Wang, Youqing Xiang, and Inna Novak; as well as Masaaki Komatsu and Keiji Tanaka of the Tokyo Metropolitan Institute of Medical Science, Bunkyo-ku, Tokyo, Japan.
Source: Albert Einstein College of Medicine















