Reverse Chemical Switching of a Ferroelectric Film
(PhysOrg.com) -- Ferroelectric materials display a spontaneous electric polarization below the Curie temperature that can be reoriented, typically by applying an electric field. In this study, researchers from Argonne, Northern Illinois University, and The University of Pennsylvania have demonstrated that the chemical environment can control the polarization orientation in an ultrathin ferroelectric film.
This is complementary to recent predictions that polarization can affect surface chemistry and illuminates potential applications in sublithographic patterning and electrically tunable catalysts.
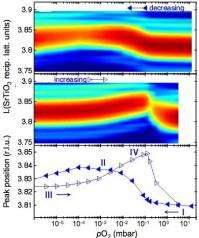
In situ synchrotron X-ray scattering measurements showed that high or low oxygen partial pressure induces outward or inward polarization, respectively, in an ultrathin PbTiO3 film. While X-ray scattering is not sensitive to interfacial charge from polarization, it is very sensitive to the atomic positions in the crystal structure of a ferroelectric film that determine its polarization.
The image shows hysteresis in the ferroelectric film structure as a function of oxygen partial pressure indicating polarization switching. The most intense (red) feature is the PbTiO3 Bragg peak. By following the behavior in situ, one sees that chemical potential affects ferroelectric film polarization in the same way as electric potential. In combination with ab initio based modeling, these experiments show that the chemical environment can play a dominant role in the behavior of nanoscale ferroelectrics.
More information:
• Wang et al., "Reversible Chemical Switching of a Ferroelectric Film," Phys. Rev. Lett., 102, 047601 (2009),
• J. Hinka, "A Viewpoint on Reversible Chemical Switching of a Ferroelectric Film," Physics. 2, 8 (2009) (online)
Provided by Argonne National Laboratory