Process for expansion and division of heart cells identified
Researchers at the Gladstone Institute of Cardiovascular Disease (GICD) and the University of California, San Francisco have unraveled a complex signaling process that reveals how different types of cells interact to create a heart. It has long been known that heart muscle cells (cardiomyocytes) actively divide and expand in the embryo, but after birth this proliferative capacity is permanently lost.
How this transition occurs has not been known. In the current issue of the journal Developmental Cell, the scientists show that the secret to this switch lies in the cells that surround the muscle cells, known as fibroblasts, which send signals that tell cardiomyocytes to divide or get bigger in size. Manipulation of these signals may be able to induce cardiomyocytes to divide again for regenerative purposes after heart attacks.
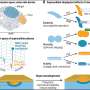
Cells exist in a three-dimensional matrix with other cells. Reciprocal signaling between the neighboring cells, along with internally generated factors and signals, insure that the tissues attain the correct shape, size, and function. In heart development, prior to birth (embryogenesis) heart cells (cardiomyocytes) proliferate and develop into different parts of the heart. After birth, the cells no longer proliferate. Although they continue to grow, the inability to proliferate renders the heart unable to regenerate cells after they have been damaged as occurs in heart attacks. It has been suggested that cardiac fibroblasts, which are cells that surround the muscle cells and make up over half of the heart cells, might be important in embryogenesis, but little is known about their development and roles in the embryonic heart.
"We've always suspected that different cell types are involved in determining how a heart is built," said GICD Director and senior author Deepak Srivastava, MD. "Our research showed that the signals from cardiac fibroblasts contribute to the different responses of cardiomyocytes."
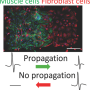
To replicate the cell interactions in the developing heart, the scientists developed a novel method of growing two distinct cell types together. By observing the cells in this system, they found that the embryonic cardiac fibroblasts promoted cell division by the cardiomyocytes more efficiently than adult cardiac fibroblasts. Furthermore, they found that fibronectin, collagen and a heparin-binding EGF-like growth factor are secreted specifically by the embryonic cardiac fibroblasts as signals to promote this cell division. These molecules act through another signaling molecule called b1 integrin, found on the surface of cardiomyocytes. The team confirmed their observations in mutant mice that lacked b1 integrin. The mutant mice had fewer myocardial cells and disruptions of the muscle integrity that eventually led to prenatal death.
"We found a major difference in the function of embryonic and adult cardiac fibroblasts. Embryonic cardiac fibroblasts promote myocyte proliferation, while adult fibroblasts promote myocyte hypertrophy," said Masaki Ieda, MD, PhD, Gladstone postdoctoral fellow and lead author on the study. "We are now trying to make adult cardiac fibroblasts more like their embryonic counterparts to induce cardiomyocyte proliferation in the adult."
"Fibroblasts are also abundant and integrally involved in many other tissues, including skin, breast, lung and some cancers. Our results may be relevant to the broader understanding of tissue development, function, and disease," said Dr. Srivastava.
Source: Gladstone Institutes