January 20, 2009 feature
'Core-Shell' Silicon Nanowires May Improve Lithium-Ion Batteries
(PhysOrg.com) -- Researchers have found a way to incorporate silicon into the structure of rechargeable lithium-ion batteries, which are used to power a wide variety of portable electronic devices, including digital cameras and cell phones. The group's method, using a nanowire form of silicon, overcomes the roadblocks that have prevented the use of silicon and may help extend the batteries' lifetimes.
Lithium ion batteries work are based on the movement of lithium ions between two battery terminals, the anode and cathode. The ions are stored in the anode, nestled between the layers of the anode material, which is often graphite. When they discharge, the ions move to the cathode.
One advantage of a graphite anode is the small volume change that occurs when the ions enter it. Additionally, existing lithium-ion batteries do boast fast ion movement rate between the terminals. But, despite their success, the batteries have a limited charge storage capacity and are not expected to be able to meet the needs of new technologies, which demand higher charge storage and longer battery life.
Silicon has been eyed as a material that can allow researchers to overcome challenges, but there have been problems making it work. Silicon expands too much during ion insertion, for example, and bulk silicon breaks and loses capacity too quickly.
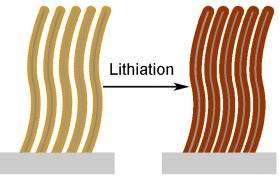
Researchers from Stanford University seem to have overcome these issues using a nanostructured form of silicon. As described in the December 23, 2008, online edition of Nano Letters, they created silicon nanowires with a "core-shell" structure, consisting of a center solid wire surrounded by a cylindrical shell, similar to a coaxial cable. The core is crystalline while the shell has a disordered, or "amorphous," structure. This works builds upon a result they published in January 2008 in Nature Nanotechnology, where they reported using a single-crystal nanowire to achieve a charge storage capacity ten times that of carbon.
"The crystalline and amorphous components have separate qualities that make the overall wires successful as a battery anode material," said the study's corresponding researcher Yi Cui, a materials scientist at Stanford, to PhysOrg.com. "We thought it might be possible to use the amorphous shell to store the ions, while the core would provide mechanical support and an efficient electron conduction pathway."
Both crystalline and amorphous silicon can store lithium ions similarly well, but amorphous silicon seems to perform better over many cycles. It also reacts with lithium at a higher electric potential, a convenient way of making ion storage the exclusive job of the amorphous shell. If the potential is maintained at the higher level, lithium ions cannot be stored in the core.
Core-shell silicon nanowires have been incorporated into other technologies, such as solar cells, but not before in batteries. Cui and his colleagues found that the amorphous shell does expand when limiting the charging potential, but not significantly. And the wires have a high charge-storage capacity—about three times that of carbon—and retain the capacity at the 90% level over 100 charge-discharge cycles. The core-shell nanowire design enables a very fast cycle, about seven minutes, and can provide a very large amount of power.
Citations:
1. Nano Lett., Article ASAP DOI:10.1021/nl8036323
2. Nature Nanotechnology, 2008, vol 3, p 31, DOI:10.1038/nnano.2007.411
Copyright 2007 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.