Researchers find new molecule to block ‘Hedgehog’ signaling in cancer, development
(PhysOrg.com) -- Researchers have achieved a feat drug developers had thought difficult, if not impossible, discovering a compound that blocks the functioning of a key developmental protein by binding to an “undruggable” target — an advance that may provide a new avenue to fight skin, pancreatic, prostate, and other cancers.
A team led by Stuart Schreiber, the Morris Loeb Professor of Chemistry in Harvard’s Faculty of Arts and Sciences and director of the Broad Institute of Harvard and MIT’s Chemical Biology Program, discovered a small molecule they dubbed “robotnikinin” that binds directly to a protein called Sonic Hedgehog.
The protein was shown in 1978 to control the early development of fruit flies, whose bodies developed spiny protrusions when the protein was blocked. It is now known that Sonic Hedgehog plays an important developmental role in mammals, including humans, regulating limb and brain development in the embryo and controlling the division both of adult stem cells and several types of cancer cell.
The new research, described in today's online edition of the journal Nature Chemical Biology, is significant not just because it provides a new way to block the actions of the Hedgehog protein, but also because it shows that there are small molecules — potential drug candidates — effective against the vast catalog of human genes currently considered “undruggable,” Schreiber said.
The human body’s functioning relies on the work of different kinds of proteins, such as enzymes and hormones, which trigger or play key roles in chemical chain reactions.
Drug discovery depends on understanding the chain reactions altered by disease and finding compounds that can affect those reactions in some way. Though the proteins involved in these reactions may seem like natural drug candidates, proteins are long, complex molecules that can be difficult to manufacture and administer in pill or liquid form. Drug discovery often, therefore, relies on the ability of chemists and pharmaceutical researchers to find smaller molecules — easy to manufacture and administer to patients — with properties that mimic, alter, or block the effects of those proteins.
Some types of proteins have proven easier to target with small molecules than others, Schreiber said, providing drug developers with a catalog of about 450 “druggable” targets. That means the proteins produced by the vast majority of the 20,000 genes in the human genome are considered “undruggable,” a fact that closes off a wide array of potential treatments and avenues of disease exploration.
The Hedgehog protein had until now been considered “undruggable,” Schreiber said, though earlier work had identified a “druggable” target further down the chemical chain reaction — called a “signaling pathway” — that it triggers. Phase 1 clinical trials are under way with two compounds that block the pathway through that previously identified target.
Finding small molecules that affect these “undruggable” targets known to be involved in disease processes is the main goal of the Broad’s Chemical Biology Program, Schreiber said. “This study is representative of exactly the type of study we’re undertaking in a general sense. What we’re trying to do with chemical biology at the Broad is to develop small molecule probes against causal disease genes — including targets and processes believed to be impossible to modulate with small molecules,” Schreiber said. “The view we have is it’s not impossible, but it takes new and different approaches.”
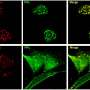
The research team, which included 14 scientists from the Broad, Harvard’s Departments of Chemistry and Chemical Biology and of Stem Cell and Regenerative Biology, Massachusetts General Hospital, and Stanford Medical School, screened 10,000 small molecule candidates using a small molecule microarray. The microarray consisted of tiny samples of the compounds arranged on a microscope slide that was then exposed to Hedgehog and examined to see which compounds reacted. The most promising candidates were then used in subsequent tests. Robotnikinin blocked the Hedgehog signaling pathway in both human skin cells and in synthetic human skin tissue.
Schreiber said there’s no reason to believe that using robotnikinin to block the Hedgehog protein directly is a better way to block the Hedgehog signaling pathway than the method currently in clinical trials, but it does provide a second approach, should the current trials not work.
Schreiber said their work with robotnikinin is continuing. His team has begun testing the compound in animal models and has seen encouraging evidence of its effectiveness. Many steps remain, however, before robotnikinin or a compound based on it would be useful in human cancers or developmental diseases, Schreiber said.
Provided by Harvard University