Fatty liver disease medication may have no effect
A new randomized, prospective trial has shown that orlistat, a commonly prescribed inhibitor of fat absorption, does not help patients with fatty liver disease (FLD) lose weight, nor does it improve their liver enzymes or insulin resistance. These findings are in the January issue of Hepatology, a journal published by John Wiley & Sons on behalf of the American Association for the Study of Liver Diseases (AASLD).
With obesity on the rise throughout the world, FLD has become a major epidemic. Weight loss improves liver enzymes and levels of fat in the liver, and the study did show that patients who lost 5 percent or more of their body weight over nine months did experience these improvements.
However, since it is often difficult for people to lose weight through diet and exercise, researchers have looked for medications that could help. Many studies have focused on orlistat, which is sold as the brand-name Xenical and over-the-counter as Alli.
Researchers led by Stephen A. Harrison, a U.S. Army hepatologist, conducted a randomized, controlled trial of overweight patients with FLD to determine the effect of orlistat in conjunction with caloric restriction. They included 50 people who had been diagnosed with FLD after clinical evaluation and liver biopsy. For 36 weeks, all subjects followed a diet of 1400 calories per day, a multivitamin and vitamin E regimen and were randomized to take orlistat (120 mg orally, three times per day with meals).
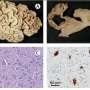
After 36 weeks, patients underwent a liver biopsy and the researchers looked for improvement in fat levels and fibrosis score. They also monitored changes in biochemical data such as fasting insulin and glucose, liver enzymes, lipid panel vitamin E and free fatty acid levels.
"Comparing the orlistat group to the non-orlistat group at study completion, no significant differences were identified between the two groups for mean weight loss, serum, insulin resistance or cholesterol," the authors report. In addition, there were no significant differences in the liver biopsy findings.
Because there were no notable differences between the two groups, the researchers reanalyzed the data to compare subjects who lost differing amounts of body weight. They noted a linear relationship between weight loss and liver improvement. In fact, body weight loss of 9 percent or more resulted in the greatest amount of liver improvement.
"In conclusion, while this preliminary study does not demonstrate a weight loss advantage with the use of orlistat," the authors report, "it does demonstrate that moderate weight loss is associated with significant improvements in the symptoms of FLD."
Article: "Orlistat for Overweight Subjects with Nonalcoholic Steatohepatitis (NASH): A Randomized, Prospective Trial." Harrison, Stephen; Fecht, William; Brunt, Elizabeth; Neuschwander-Tetri, Brent. Hepatology ; January 2009.
Source: Wiley