Findings turn events in early TB infection on their head, may lead to new therapy
Masses of immune cells that form as a hallmark of tuberculosis (TB) have long been thought to be the body's way of trying to protect itself by literally walling off the bacteria. But a new study in the January 9th issue of the journal Cell, a Cell Press publication, offers evidence that the TB bacteria actually sends signals that encourage the growth of those organized granuloma structures, and for good reason: each granuloma serves as a kind of hub for the infectious bugs in the early stages of infection, allowing them to expand further and spread throughout the body.
"This fundamentally turns our understanding of granulomas all topsy turvy," said Lalita Ramakrishnan of the University of Washington, Seattle. "Scientists thought they were protective, but they are not—at least not in early infection. The bacteria use them to reproduce and disseminate themselves."
Not only do the bacteria expand themselves within the first granuloma to form, she added, but some of the immune cells in that initial mass leave to start new granulomas elsewhere. Those new granulomas then also serve as breeding grounds for the bacteria.
The finding suggests a new avenue for TB therapy at an important time in the struggle against TB infection. "We might think about ways to prevent granulomas that might be therapeutic," Ramakrishnan said. That might be done either by intercepting the bacterial signal that spurs granulomas' formation or by manipulating the human immune system in some other way.
"Finding a new way to intervene in the infection is particularly relevant now because there is a horrible epidemic of drug-resistant TB," she added. "Many of the bugs are resistant to practically everything."
At the outset of human pulmonary tuberculosis, the inhaled bacteria (Mycobacterium tuberculosis) is gobbled up by immune cells known as macrophages and transported into the lung. There, infected macrophages recruit additional macrophages and other immune cells to form granulomas. Under the classical view, those granulomas help protect against the bacteria, even if they don't successfully contain the infection. They were also thought to form only after the adaptive immune system shifts into gear.
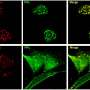
But Ramakrishnan's team began to find evidence calling that classical view into question by studying the disease in zebrafish embryos. Because zebrafish embryos are transparent, they allowed the team to literally watch the infection take hold and spread in real time.
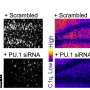
Their initial studies showed that, contrary to the classical view, granulomas form well before adaptive immunity comes into play, within days of infection. Indeed, granulomas' formation coincides with the bacteria's expansion. In addition, in embryonic fish infected with a less-virulent, mutant strain of bacteria, which lacked a secretion system known as ESX-1/RD1, granulomas didn't form nearly as well. Together, those findings suggested to Ramakrishnan's team that granuloma formation actually works not as a protective maneuver on the part of the infected host, but rather as a bacterial tool for expanding infection.
To further investigate in the new study, the researchers observed and quantified the events in zebrafish embryos infected with normal TB bacteria and the mutant bacteria lacking the ESX-1/RD1 system. They found that, once transported inside of cells by macrophages, the bacteria use the RD1 signal to call on new macrophages to come and move in to the growing granuloma. As multiple macrophages arrive, they efficiently find and consume infected and dying macrophages to become infected themselves. That process leads to a rapid, iterative expansion of infected macrophages and thereby bacterial numbers, they report. The primary granuloma also seeds secondary granulomas as infected macrophages leave for other parts of the body.
"In summary," the researchers wrote, "we propose that the pathway of granuloma formation and subsequent bacterial dissemination is based upon macrophage responses that are of themselves generally protective and that work reasonably well against less virulent (i.e., RD1-deficient) infection. Rather than block these host responses, RD1-competent mycobacteria appear to accelerate them to turn the granuloma response into an effective tool for pathogenesis. The initiation of the adaptive immune response then may halt bacterial expansion not by forming granulomas as suggested by the classical model but by altering the early granuloma into a form of stalemate between host and pathogen."
Source: Cell Press