New bone implant technology using techniques normally used to make catalytic converters
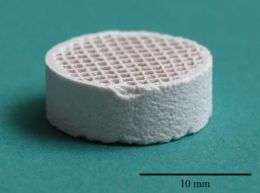
A method of producing synthetic bone, using techniques normally used to make catalytic converters for cars, is being developed by researchers at WMG at the University of Warwick.
The team is now working closely with Warwick Ventures, the University's technology transfer office, to find a suitable partner to help commercialise the technology, and will be presenting their work on 9 December at the national university technology showcase event, Bioversity.
WMG's Dr Kajal Mallick is developing the technique along with his postgraduate researcher James Meredith. They strongly believe it could offer substantial clinical benefits to patients undergoing bone implant surgery.
The technique involves state-of-the-art extrusion of the implant material through a mould, to produce a 3-dimensional honeycomb texture, with uniform pores throughout. The material can then be sculpted by the surgeon to precisely match the defect. After implantation bone cells will be transported into the implant and begin to form new bone.
"We worked with a Japanese company which manufactures catalytic converters and used their facility to produce samples which we could then test in the laboratory," explains Dr Mallick.
"We found that we were able to use calcium phosphates – a family of bioceramics that are routinely used in bone implant operations, but by using this technique we were able to improve significantly both the strength and porosity of the implant."
Dr Mallick added: "At the present time, there is no product available in the market place that satisfies both these key properties simultaneously. It is nearly an ideal scaffold structure for efficient blood flow and formation of new bone cells."
The increased strength of the material means it could be used in spinal surgery, or in revision hip and knee operations, where currently non-degradable materials such as titanium or steel may be used. The advantage of increased and interconnected porosity is that the implant can quickly be filled with blood vessels, resulting in a more rapid healing process.
James Meredith is working to complete an Engineering Doctorate in this research area. He says: "The synthetic bone we are developing is as strong as normal healthy bone yet porous enough to allow bone cells to inhabit it and generate new bone. Over a period of time, we expect the synthetic bone will resorb, leaving only natural bone. I hope that if we can find an industrial partner to take this to market, we will enable treatment of conditions which up to this point have only been possible using metal replacement parts or low strength foam-like bone substitutes."
Source: University of Warwick





















