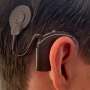
MRI machines may damage cochlear implants
Patients with cochlear implants may want to steer clear of certain magnetic imaging devices, such as 3T MRI machines, because the machines can demagnetize the patient's implant, according to new research published in the December 2008 issue of Otolaryngology – Head and Neck Surgery.
A cochlear implant is an electronic device that restores partial hearing to the deaf. It is surgically implanted in the inner ear and activated by a device worn outside the ear. Unlike a hearing aid, it does not make sound louder or clearer. Instead, the device bypasses damaged parts of the auditory system and directly stimulates the nerve of hearing, allowing individuals who are profoundly hearing impaired to receive sound. It is estimated that more than 100,000 people have cochlear implants.
The study, conducted by a team of German and American researchers, tested several cochlear device magnets on a 3T MRI scanner with active shielding at a variety of angles (0º, 80º, 90º, 100º, 110º, and 180º). The researchers discovered that during routine use of 3T MRI machines at angles above 80º, an unacceptable level of demagnetization was reached, causing permanent damage to devices with non-removable magnets, and creating the potential of exposing patients to undesirable magnetic forces.
3T MRI scanners are the next generation of MRI scanners and are significantly more powerful than 1.5T MRI scanners.
As a result of their findings, the study authors recommend that MRI scans on patients with cochlear implants should be performed using a 3T MRI machine only if a 1.5T machine is not available, and if the benefits of the scan far outweigh the risk of cochlear implant demagnetization.
Source: American Academy of Otolaryngology