New promising obesity drug may have huge potential
According to trials, a new obesity drug, Tesofensine, which may be launched on the world market in a few years, can produce weight loss twice that of currently approved obesity drugs. The Danish company Neurosearch and a number of researchers at the Faculty of Life Sciences at University of Copenhagen are behind the promising findings.
Tesofensine can produce weight loss twice that of currently approved obesity drugs, and should be studied in phase III trials. These are the conclusions of an Article published early Online and in an upcoming edition of The Lancet, written by Professor Arne Astrup, Department of Human Nutrition, Faculty of Life Sciences, University of Copenhagen, Denmark, and colleagues.
Increased obesity prevalence worldwide, in both developed and developing countries, results in more people with cardiovascular disease, diabetes, musculoskeletal disorders, and cancer. Whilst gastric bypass surgery substantially reduces bodyweight and obesity-related disease, the researchers believe a treatment gap exists between the effectiveness of currently marketed obesity drugs and gastric-bypass surgery. Tesofensine – which inhibits the presynaptic uptake of the neurotransmitters noradrenaline, dopamine and serotonin in the brain – has been shown to be safe and effective in animal models. It also caused unintended weight loss when it was given obese patients with Parkinson's or Alzheimer's disease when it was researched for those conditions. The drug works by suppressing hunger, leading to an energy deficit which burns off excess body fat.
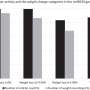
This randomised, placebo-controlled phase II study was done in five Danish obesity management centres, and involved 203 obese patients (body mass index 30-40 kg/m2), weighing a mean of just over 100kg. They were prescribed a limited-energy diet and assigned to tesofensine 0.25mg (52 patients), 0.5 mg (50), 1.0 mg (49), or placebo (52), all once daily for 24 weeks. The primary outcome was percentage change in bodyweight. A total of 161 patients completed the study, and an analysis showed that the mean weight loss recorded for placebo and diet was 2.2kg and for tesofensine 0.25mg, 0.5mg and 1.0mg it was 6.7kg, 11.3kg, and 12.8kg respectively. For the 0.5mg and 1.0mg doses, this represented a weight loss around twice that attained using sibutramine or rimonabant*, the currently-approved therapies in Europe. Blood pressure was increased in the 1.0mg group.The most common side-effects caused by tesofensine were dry mouth, nausea, constipation, hard stools, diarrhoea, and insomnia.
The authors conclude that the 0.5mg dose of tesofensine is more promising than the 1.0mg dose because it produces a similar weight loss with less side-effects. They say: "We conclude that tesofensine 0.5 mg, once daily for 6 months, has the potential to produce twice the weight loss as currently approved drugs; however, larger phase III studies are needed to substantiate our findings."
Source: University of Copenhagen