Scientists Use Light to Control Proteins
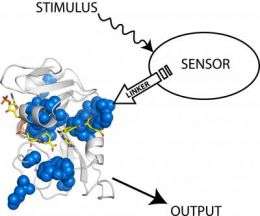
A team of researchers from Penn State and the University of Texas Southwestern Medical Center has discovered a way to use light to control certain proteins that catalyze biochemical reactions. "This is one of the first examples of someone successfully controlling the activity of a protein using light," said Stephen Benkovic, Penn State Evan Pugh Professor of Chemistry, holder of the Eberly Family Chair in Chemistry, and one of the team's leaders. "The technology one day could be expanded to have multiple uses, including the ability to turn off the activities of some disease-causing proteins in the cell," he said. The team's results will appear in the 17 October issue of the journal Science.
In their experiment, the scientists designed a hybrid protein by inserting a light-sensing protein from an oat plant into an enzyme -- a type of protein that catalyzes biochemical reactions -- from the bacterium E. coli. After engineering the two components together, the researchers found that the enzyme's activity could be manipulated by shining a light on the light-sensing protein, which the scientists refer to as a "domain." "The technology works like a light switch," said Benkovic. "When we shine a light on the light-sensing domain, the enzyme's activity increases, and when we shut the light off, the enzyme's activity decreases."
According to Jeeyeon Lee, a postdoctoral scholar in the Penn State Department of Chemistry and one of the paper's authors, the team had to consider a number of factors when designing the hybrid protein, including the protein's shape, or what is referred to as its conformation. "The conformation of a protein is important in determining its function," she said. "Without the proper conformation, our protein would not have responded to the light."
Another important factor that the team had to consider was the proper location on the enzyme into which the light-sensing protein from the oat plant would be inserted. Vishal Nashine, also a postdoctoral scholar in the Department of Chemistry and an author of the paper, said the team was surprised to find that the switch worked only when they attached the light-sensing domain to the enzyme at a particular site. The switch did not operate when they attached the molecule to other locations on the enzyme. "The fact that the switch worked only when the light-sensing domain was attached to the enzyme at a specific site suggests that a unique network is active at that site through which signals, such as those responding to light, are transmitted," he said.
The successful site was located, among more than a hundred different possibilities, using a computational algorithm called the Statistical Coupling Analysis (SCA), which was pioneered by Rama Ranganathan, a professor of pharmacology at the University of Texas Southwestern Medical Center and one of the paper's leading authors.
The team's future research will investigate how the signal triggered by the light was transmitted from the light-sensing domain to the enzyme. "It is not yet clear how this process works," said Benkovic. "So far, the effect has been small, but we plan to optimize the technology to see if we can use light to modulate the enzyme's activity in alternative ways."
Source: Penn State