A key mechanism regulating neural stem cell development is uncovered
A research team at the Institut de recherches cliniques de Montreal (IRCM), funded by the Foundation Fighting Blindness – Canada and the Canadian Institutes of Health Research (CIHR), discovered a novel mechanism that regulates how neural stem cells of the retina generate the appropriate cell type at the right time during normal development. These findings, published today in the renowned journal Neuron, could influence the development of future cell replacement therapies for genetic eye diseases that cause blindness.
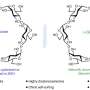
In their report, the scientists show that a gene called Ikaros is expressed in the most immature retinal stem cells in the mouse, which are "competent" to generate all seven different cell types that compose the retina. But this gene is not expressed in the "older" stem cells, which are more restricted in their differentiation potential and produce only the late-born neurons.
"By studying the retina of a mouse in which the Ikaros gene was inactivated, we found that the generation of early-born retinal cell types was impaired, whereas the generation of the late-born retinal cell types was not affected," explained Dr. Michel Cayouette who led the study. In contrast, forcing the expression of Ikaros in older retinal stem cells, which have normally turned off its expression, was sufficient to give back the competence to these cells to generate early-born neurons. Overall, these results indicate that the expression of Ikaros in retinal stem cells is both necessary and sufficient to confer the competence to generate early-born retinal neurons.
The identification of adult retinal stem cells in recent years has opened up the possibility that such cells could one day be used to replace damaged or lost cells in various retinal diseases such as glaucoma, macular degeneration or retinitis pigmentosa. For such approaches to be effective, however, it is crucial that stem cells generate only the appropriate cell type for a particular condition.
This study suggests that it may be possible to manipulate the competence of retinal stem cells so that they only generate retinal cells associated to a particular temporal stage. "For example, added Dr. Cayouette, inactivating Ikaros could favor the production of later-born neurons such as photoreceptors, which are lost progressively in retinal degenerative diseases." Future studies will be required to assess the usefulness of this approach for potential cell replacement therapies.
Source: Institut de recherches cliniques de Montreal