First atomic–scale compositional images of fuel-cell nanoparticles
(PhysOrg.com) -- In a step toward developing better fuel cells for electric cars and more, engineers at MIT and two other institutions have taken the first images of individual atoms on and near the surface of nanoparticles key to the eco-friendly energy storage devices.
Nanoparticles made of platinum and cobalt are known to catalyze some of the chemical reactions behind fuel cells, making those reactions run up to four times faster than if platinum alone is used as the catalyst.
No one, however, understands exactly why. That’s because “little is known about the nanoparticles’ surface atomic structure and chemistry,” which are key to the particles’ activity, said Yang Shao-Horn, an associate professor in the Department of Mechanical Engineering and Department of Materials Science and Engineering and director of the Electrochemical Energy Laboratory at MIT.
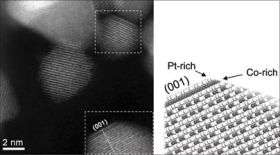
Using a new technique known as aberration-corrected Scanning Transmission Electron Microscopy, Shao-Horn’s team, in collaboration with Professor Paulo Ferreira of the University of Texas at Austin and Dr. Larry Allard of Oak Ridge National Laboratory, identified specific atomic structures near the surface of such a catalyst. That information in hand, the researchers propose a theory for why the material is so active. Perhaps most importantly, “knowing the surface composition will help us design even better catalysts,” Shao-Horn said.
The work was reported in the Sept. 24 online issue of the Journal of the American Chemical Society.
The researchers analyzed platinum and cobalt nanoparticles that were either treated with acid, or treated with acid then subjected to high heat. Nanoparticles produced both ways are known to be more active than platinum alone. Shao-Horn and colleagues found that each, in turn, also had slightly different surface structures.
For example, in the nanoparticles subjected to heat treatments, the platinum and cobalt atoms formed a “sandwich-like” structure. Platinum atoms covered most of the surface, while the next layer down was composed primarily of cobalt. Successive layers contained mixtures of the two.
The team proposes that these particular nanoparticles are up to four times more active than platinum alone because the platinum atoms on the surface are constrained by the cobalt atoms underneath. “This modifies the interatomic distances between the platinum atoms on the nanoparticle surface,” making them more effective in chemical reactions key to fuel cells, Shao-Horn said.
She further noted that “this work bridges the gap between our understanding of electrocatalysis in bulk materials and at the nano-scale.”
In addition to Shao-Horn, Allard, and Ferreira, who is also an MIT research affiliate, other members of the research team are Shuo Chen, first author of the paper and a postdoctoral associate in mechanical engineering; Wenchao Sheng, a graduate student in chemistry; and Naoaki Yabuuchi, a research affiliate in mechanical engineering.
The Department of Energy and the National Science Foundation, through its Materials Research Science and Engineering Center program, funded the work.
Provided by MIT




















