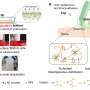
Site used by sodium to control sensitivity of certain potassium ion channels
Virginia Commonwealth University School of Medicine researchers have uncovered how sodium is able to control specific potassium ion channels in cells, according to new study findings published online this week in Nature Chemical Biology.
These findings, published Sept. 14, may help researchers gain a greater understanding of the mechanisms involved in ion channel gating and may one day set the stage for new approaches in drug design.
Intracellular-sodium is known to control the opening and closing of certain potassium channels. Using a computational-experimental approach, researchers examined the interaction between sodium and a group of potassium channels known as Kir channel proteins. The team had previously shown that Kir3 channels are sensitive to sodium, but had not shown how sodium is coordinated by specific amino acids found in several Kir channel proteins.
"We have a fairly good molecular understanding of how the sodium ions are coordinated and we have a compelling hypothesis of how the coordination of sodium may be affecting this particular type of potassium ion channel to open it," said Diomedes Logothetis, Ph.D., an internationally recognized leader in the study of ion channels and chair of the VCU School of Medicine's Department of Physiology and Biophysics.
According to Logothetis, ion channel proteins are found in the plasma membrane of cells and provide a path for hydrated cations such as calcium, potassium or sodium to pass through the membrane and travel in and out of the cell. Generally, hydrated ions do not travel through the lypophillic, or fat-based, plasma membrane because they are surrounded with water and require a pathway to travel from one side of the membrane to the other.
"By applying the knowledge of the sodium coordination site, we identified the Kir5.1 channel that was not previously suspected to be sodium-sensitive. By examining in which cells these sodium-sensitive channels are expressed and how sodium may be critical for the function of these cells, we hope to uncover the physiological importance of sodium-mediated activation of potassium channels," Logothetis said. Cells in the kidney and specific areas in the brain express the Kir5.1 channel, he added. In kidney cells, the Kir channel is expressed in cells that are responsible for reabsorbing sodium, a process relevant in hypertension as sodium and water are retained in blood vessels.
One critical characteristic of the sodium-reabsorbing kidney cells is that they maintain a negative-membrane potential, meaning the inside is more negative than the outside, which creates a driving force for sodium entry into the cells. This negative membrane potential is accomplished by potassium flowing out of the cell through potassium channels.
If Kir5.1 is linked to sodium reabsorption, then malfunctions of this channel could interfere with the sodium uptake mechanisms from blood vessels through the kidney cells and lead to hypertension. Logothetis and his collaborators in New York and Oxford, England, are in the process of testing this hypothesis.
"Once we understand the importance of sodium regulation of ion channels, then we can start thinking about how we design drugs that may mimic the net effect of sodium regulation of channel activity and ultimately help any pathophysiology dependent on these processes," he said.
Source: Virginia Commonwealth University