Researcher develops novel method to grow human embryonic stem cells
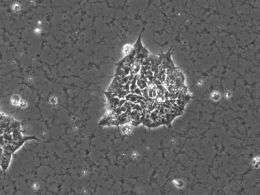
(PhysOrg.com) -- The majority of researchers working with human embryonic stem cells (hESCs) – cells which produce any type of specialized adult cells in the human body – use animal-based materials for culturing the cells. But because these materials are animal-based, they could transmit viruses and other pathogens to the hESCs, making the cells unsuitable for medical use.
Now, a stem-cell scientist at UC Riverside has devised a method of growing hESCs in the lab that uses no animal-derived materials – an important advance in the use of hESCs for future medical purposes.
Because of their tremendous potential, hESCs are considered promising sources for future cell therapy to treat diseases such as Parkinson's disease and diabetes mellitus.
Noboru Sato, an assistant professor of biochemistry, developed the new method, which is not only cleaner and easier to use than conventional methods of culturing hESCs but also results in hESCs whose pluripotency – the potential to differentiate into any of the specialized cells of the body such as neurons, cardiac muscles, and insulin-producing cells – is uncompromised.
Currently in labs worldwide, many researchers grow hESCs on Matrigel-coated culture plates, Matrigel being the trade name for a gelatinous extract, taken from mouse tumor cells, that contains extracellular matrices (ECMs), made up of special proteins. The Matrigel coating provides the scaffolding to which the hESCs first attach and then grow in undifferentiated colonies before differentiating into specialized cells.
"The development of animal-free coating methods for hESCs still remains a major challenge due to the complexity of ECMs and insufficient knowledge about how hESCs control cell-cell and cell-ECM interactions," explained Sato, who led the research project.
His lab identified a specific signaling pathway, called Rho-Rock, which the hESCs use during colony formation and which plays an important role in physical interactions between hESCs. When the researchers blocked the pathway, they found, as expected, that the normal colony formation of hESCs was considerably impaired. They also found that the hESCs maintained their pluripotency.
"Until now, it was generally assumed that the hESC colony formation was pivotal for maintaining pluripotency," Sato said. "But we show that pluripotency can be retained independent of close cell-cell contact."
Prue Talbot, the director of UCR's Stem Cell Center of which Sato is a member, noted that Sato's discovery could affect the way embryonic stem cells are grown in the future.
"His work is certainly an important step forward in both understanding signal transduction pathways in stem cells and in the development of an improved methodology for culturing stem cells," she said.
Study results appear online in the Aug. 20 issue of the Public Library of Science (PLoS) ONE.
In the study, Sato's group extensively screened various types of scaffold materials in combination with Y27632, a chemical compound that blocks the Rho-Rock pathway, and found that the Matrigel coating could be replaced with "poly-D-lysine," a chemically synthesized ECM. The major advantages of poly-D-lysine over Matrigel are that poly-D-lysine is completely animal-free, easy to handle, and its quality is consistent.
"We found that the growth of the hESCs under this novel culture condition was almost identical to the growth of hESCs on Matrigel-coated culture plates, with no compromise in pluripotency," Sato said.
Having started his career as a physician in Japan, Sato began researching stem cell biology as a research fellow at The Rockefeller University, NY, one of the foremost research centers in the world. He accepted a faculty position in the Department of Biochemistry at UCR in 2006. He was joined in the research project by Nicole Harb of UCR and Trevor K. Archer of the National Institute of Environmental Health Sciences (NIEHS), NC.
The research was a collaboration between UCR and NIEHS, and funded by UCR start-up funds to Sato and a grant to Archer from the National Institutes of Health.
"Our research goal is to understand the basic mechanisms underlying unique biological functions of pluripotent stem cells, and to translate the obtained knowledge into future medical applications," Sato said.
His group is now focusing on applying his technique to the latest stem cell technology, "induced pluripotent stem (iPS) cells," which are pluripotent stem cells artificially derived from adult cells without using embryos. "Our next step is to produce new animal-free iPS cell lines," Sato said.
Office of Technology Commercialization has applied for a patent on Sato's discovery and is looking for industrial partners interested in further developing it.
Background information on stem cells:
Stem cells, which can transform themselves into many other tissue types, give rise to all the cells in the human body and hold the key to finding cures for many diseases, such as Parkinson's, Alzheimer's, heart disease and diabetes. These master cells are found in the body at any age, acting as the root of all the cells that make up the body's tissues.
When a stem cell divides, each new cell has the potential to either remain a stem cell or become a specialized cell, such as a muscle cell, a red blood cell, or a brain cell. Stem cells can theoretically divide without limit to replenish other cells as long as the person or animal is still alive. Scientists believe, therefore, that it should be possible to turn stem cells into a "repair kit" for the body.
Human fetal tissue provides the best source of stem cells (embryonic stem cells). Stem cells also are found within adult organs (adult stem cells), but currently their potential to become other types of cells is limited.
The latest exciting discovery is that normal adult cells such as skin fibroblasts can be turned to pluripotent embryonic stem-like cells by introducing key genes involved in pluripotency. The pluripotent stem cells generated by this technique are called induced pluripotent stem (iPS) cells.
Provided by University of California - Riverside