Synthetic Molecules Could Add Spice To Fight Against Cancer
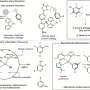
Seeking to improve on nature, scientists used a spice-based compound as a starting point and developed synthetic molecules that, in lab settings, are able to kill cancer cells and stop the cells from spreading. The researchers are combining organic chemistry, computer-aided design and molecular biology techniques in developing and testing pharmaceutical compounds that can fight breast and prostate cancer cells. The synthetic molecules are derived from curcumin, a naturally occurring compound found in the spice turmeric.
Seeking to improve on nature, scientists used a spice-based compound as a starting point and developed synthetic molecules that, in lab settings, are able to kill cancer cells and stop the cells from spreading.
The researchers are combining organic chemistry, computer-aided design and molecular biology techniques in developing and testing pharmaceutical compounds that can fight breast and prostate cancer cells. The synthetic molecules are derived from curcumin, a naturally occurring compound found in the spice turmeric.
Centuries of anecdotal evidence and recent scientific research suggest curcumin has multiple disease-fighting features, including anti-tumor properties. However, when eaten, curcumin is not absorbed well by the body. Instead, most ingested curcumin in food or supplement form remains in the gastrointestinal system and is eliminated before it is able to enter the bloodstream or tissues.
"Newer evidence describes how curcumin interacts with certain proteins to generate anti-cancer activity inside the body. We're focusing on the pathways that are most involved in cancer and trying to optimize for those properties," said James Fuchs, assistant professor of medicinal chemistry and pharmacognosy at Ohio State University and principal investigator on the project.
Fuchs presented the research today at the American Chemical Society meeting in Philadelphia. He described a selection of the 40 compounds developed to date, emphasizing the synthetic molecules that appear to have the most potential to serve as the basis for anti-cancer drug development.
Fuchs and colleagues are continuing to refine compounds that are best structured to interact with a few overactive proteins that are associated with cell activity in breast and prostate cancers. Blocking these molecular targets can initiate cell death or stop cell migration in the cancers.
A major component of their strategy is called structure-based, computer-aided design, a relatively new technology in the drug discovery field. Before ever working with an actual compound, the scientists can make manipulations to computer-designed molecules and observe simulated interactions between molecules and proteins to predict which structural changes will make the most sense to pursue.
"Most of the interaction between our compound and the overactive protein comes from what are called hot spots on the protein's surface," said Chenglong Li, assistant professor of medicinal chemistry and pharmacognosy at Ohio State and an expert in computational chemistry. "For each spot, we can design small chemical fragments and link them together to make a molecule. This is what computer-aided design and modeling can do."
Some of the most effective compounds have been tested for their effectiveness against human cancer cell lines – as well as whether they might be toxic to healthy cells. So far, the molecule favored by the researchers has a nearly 100-fold difference in toxicity to cancer cells vs. healthy cells, meaning it takes 100 times more of the compound to kill a healthy cell than it does to kill a cancer cell.
"Very small changes that may seem insignificant can have dramatic effects on these toxicity properties," Fuchs said. "But most of the compounds we've made have been more potent than curcumin against the cancer cells."
The computer-based predictions have suggested that the most effective compound developed to date can interact with proteins believed to be active in about 50 percent of all breast and prostate cancers.
"To be able to develop a drug that in the future could have potential to treat 50 percent of these cancers would be a major contribution," said Jiayuh Lin, an investigator in Ohio State's Comprehensive Cancer Center and an associate professor of pediatrics. Lin tests the experimental compounds in different types of breast and prostate cancer cell lines. He said some of the compounds also show potential to kill pancreatic cancer cells and inhibit cancer cell migration.
The computer-aided design also offers hints at the compounds' suitability as the basis for a drug, such as whether the molecules will remain stable during metabolism and whether they will maintain a structure that the body can absorb into the bloodstream and tissues. The team is planning to continue refining the compounds before advancing to animal studies to test their effectiveness. The scientists hope to develop a chemotherapeutic agent available in pill form.
Source: Ohio State University