Now That's Cool: Engineers Out to Thaw the Mysteries of Ice
(PhysOrg.com) -- "Ye canna change the laws of physics!" Scotty warned Captain Kirk on Star Trek. But engineers and physicists at the University of Maryland may rewrite one of them.
The Third Law of Thermodynamics is on the minds of John Cumings, assistant professor of materials science and engineering at the University of Maryland's A. James Clark School of Engineering, and his research group as they examine the crystal lattice structure of ice and seek to define exactly what happens when it freezes.
"Developing an accurate model of ice would help architects, civil engineers, and environmental engineers understand what happens to structures and systems exposed to freezing conditions," Cumings said. "It could also help us understand and better predict the movement of glaciers."
Understanding the freezing process is not as straightforward as it may seem. The team had to develop a type of pseudo-ice, rather than using real ice, in order to do it.
Despite being one of the most abundant materials on Earth, water, particularly how it freezes, is not completely understood. Most people learn that as temperatures fall, water molecules move more slowly, and that at temperatures below 32º F/0º C, they lock into position, creating a solid—ice. What's going on at a molecular level, says Cumings, is far more complicated and problematic. For one thing, it seems to be in conflict with a fundamental law of physics.
The Third Law of Thermodynamics states that as the temperature of a pure substance moves toward absolute zero (the mathematically lowest temperature possible) its entropy, or the disorderly behavior of its molecules, also approaches zero. The molecules should line up in an orderly fashion.
Ice seems to be the exception to that rule. While the oxygen atoms in ice freeze into an ordered crystalline structure, its hydrogen atoms do not.
"The hydrogen atoms stop moving," Cumings explains, "but they just stop where they happen to lie, in different configurations throughout the crystal with no correlation between them, and no single one lowers the energy enough to take over and reduce the entropy to zero."
So is the Third Law truly a law, or more of a guideline?
"It's a big fundamental question," says Cumings. "If there's an exception, it's a rule of thumb."
Materials that violated the Third Law as originally written were found in the 1930s, mainly non-crystalline substances such as glasses and polymers. The Third Law was rewritten to say that all pure crystalline materials' entropy moves toward zero as their temperatures move toward absolute zero. Ice is crystalline—but it seems only its oxygen atoms obey the Law. Over extremely long periods of time and at extremely low temperatures, however, ice may fully order itself, but this is something scientists have yet to prove.
Creating an accurate model of ice to study has been difficult. The study of ice's crystal lattice requires precise maintenance of temperatures below that of liquid nitrogen (-321 °F/-196 °C), and also a lot of time: no one knows how long it takes for ice to ultimately reach an ordered state—or if it does at all. Experiments have shown that if potassium hydroxide is added to water, it will crystallize in an ordered way—but researchers don't know why, and the addition shouldn't be necessary due to the Third Law's assertion that pure substances should be ordered as they freeze.
To overcome these problems, scientists have designed meta-materials, which attempt to mimic the behavior of ice, but are created out of completely different substances. A previous material, spin ice, was designed from rare earth elements and had a molecular structure resembling ice, with magnetic atoms (spins) representing the position of hydrogen atoms. However, it did not always behave like ice.
The Cumings group is refining a successor to spin ice called artificial spin ice, which was originally pioneered by researchers at Penn State. The newer meta-material takes the idea a step further.
"The original spin ice research went from one part of the periodic table to a more flexible one," said Cumings. "But artificial spin ice goes off the periodic table altogether."
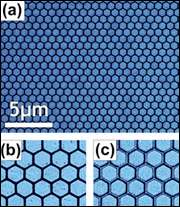
Artificial spin ice is a collection of "pseudo-atoms" made of a nickel-iron alloy. Each pseudo-atom is a large-scale model made out of millions of atoms whose collective behavior mimics that of a single one.
As with the original spin ice, magnetic fields are stand-ins for hydrogen atoms. Working at this "large" scale—each pseudo-atom is 100x30 nanometers in size (100 nanometers is 1000 times smaller than the width of a human hair)—gives the researchers control over the material and freedom to explore how real atoms behave.
"It mimics the behavior of real ice but is completely designable with specific properties," Cumings said. "We can change the strength of the spin or reformulate the alloy to change the magnetic properties, which creates new bulk properties that we either couldn't get from normal materials, or couldn't control at the atomic level."
The team is also able to image the behavior of the pseudo hydrogen atoms using an electron microscope—such direct observation is not possible with the original spin ice or real ice.
"This is the first time the rules of ice behavior have ever been rigorously confirmed by directly counting pseudo hydrogen atoms," explained group member and postdoctoral research associate Todd Brintlinger. "We can track the position and movement of each pseudo atom in our model, see where defects occur in the lattice, and simulate what happens over much longer periods of time."
The ultimate impact of the research may go beyond civil engineering and the environment. "Although we're mimicking the behavior of ice," Cumings explained, "our meta-material is very similar to patterned hard-disk media. Magnetic 'bits' used in hard drives are usually placed at random, but memory density could be increased if they were in a tight, regular pattern instead.
"We've found that both hydrogen in ice and the pseudo-hydrogen in our artificial spin ice also behave as bits, can carry information, and interact with each other. Perhaps in the future, engineers will be inspired by this in their hard drive designs. The formal patterning and bit interactions may actually help to stabilize information, ultimately leading to drives with much higher capacities."
Provided by University of Maryland