Drug has potential to prevent alcoholics from relapsing
An experimental drug that blocks the euphoric feelings associated with drinking may prevent alcoholics from relapsing. The finding, the result of a mouse study at Oregon Health & Science University, could lead to human clinical trials within the next year.
"We showed we could block behavior in mice that resembles this increased euphoria even after the animals had been given a lot of alcohol," said Tamara Phillips, Ph.D., professor and vice chair of the behavioral neuroscience department at OHSU and a research scientist at the Portland Veterans Affairs Medical Center. "That's what you want in a treatment, because we don't get to people until after they become addicted to alcohol."
Earlier research has shown that some people's brains become sensitized as a result of chronic exposure to alcohol. This change in the brain does not subside after people quit drinking. So when they begin consuming alcohol again, "they get a bigger jolt," Phillips said.
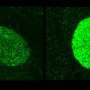
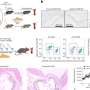
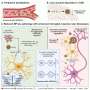
Alcohol consumption causes the body to release a substance known as "corticotrophin-releasing factor" or CRF. It activates receptors in the brain. Phillips and her team determined that a brain receptor called CRF1 appears to be involved in this heightened pleasure sensation. They compared the responses of normal mice and mice bred without the CRF1 receptor to chronic doses of alcohol. Mice without the CRF1 receptor did not experience the euphoric jolt the normal mice demonstrated.
The research team also took normal mice with the CRF1 receptor and exposed them to chronic doses of alcohol. Before testing for the euphoric response, the researchers gave the mice an experimental drug called CP 154,526 – developed by Pfizer – which prevents CRF from reaching the brain receptor. This group of mice also did not experience the heightened reaction.
Phillips' study recently was published in the Proceedings of the National Academy of the Sciences. The results may be particularly applicable to stress-induced relapse. That's because the CRF1 receptor also triggers the body's response to stress.
This could have implications for PTSD patients. "I think if you block this receptor, you might be able to decrease drinking in response to PTSD," Phillips said.
The next step is testing CP 154,526 to see if it is safe for use in humans. If it clears that hurdle, researchers will start human trials to determine if the drug can prevent alcoholic relapse.
Source: Oregon Health & Science University