Epilepsy drug may increase risk of birth defects
Taking the epilepsy drug topiramate alone or along with other epilepsy drugs during pregnancy may increase the risk of birth defects, according to a study published in the July 22, 2008, issue of Neurology, the medical journal of the American Academy of Neurology.
Research has shown that many epilepsy drugs increase the risk of birth defects, but little research has been done on topiramate. Studies have shown that topiramate increases the risk of birth defects in animals. Maintaining effective epilepsy treatment during pregnancy is crucial because seizures may cause harm to the fetus.
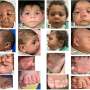
For the study, researchers examined women who became pregnant while taking topiramate either on its own or along with other epilepsy drugs. Of 178 babies born, 16 had major birth defects. Three of these were in infants whose mothers were taking only topiramate, and 13 were in those whose mothers were taking topiramate and other epilepsy drugs.
Four of the babies had cleft palates or cleft lips, a rate 11 times higher than that expected if these women were not taking epilepsy drugs. Four male babies had genital birth defects, with two of those classified as major defects, which is 14 times higher than the normal rate for this defect.
"More research needs to be done to confirm these results, especially since it was a small study," said John Craig, MRCP, of the Royal Group of Hospitals in Belfast, Northern Ireland. "But these results should also get the attention of women with migraine and their doctors, since topiramate is also used for preventing migraine, which is an even more common condition that also occurs frequently in women of childbearing age."
Craig said the risk of birth defects may be different for women taking the drug for migraine, but that the pregnancies of women exposed to topiramate should be monitored.
This study found that more birth defects occurred in women taking topiramate along with the drug valproate, or valproic acid, than in women taking topiramate and another epilepsy drug. Research has shown that valproate is associated with a high risk of birth defects.
Source: American Academy of Neurology