Potential therapy discovered for hypophosphatasia, a congenital form of rickets
Researchers at the Burnham Institute for Medical Research, led by José Luis Millán, Ph.D., have demonstrated in mice the first successful use of enzyme replacement therapy to prevent hypophosphatasia (HPP), a primary skeletal disease of genetic origin. This discovery lays the foundation for future clinical trials for HPP patients.
Rickets is a softening of the bones that most commonly results from a lack of vitamin D or calcium and from insufficient exposure to sunlight. Hypophosphatasia is a rare, heritable form of rickets caused by mutations in a gene called TNAP, which is essential for the process that causes minerals such as calcium and phosphorus to be deposited in developing bones and teeth.
The physical presentations of this disorder can vary depending on the specific mutation, with more severe symptoms occurring at a younger age of onset. The most severe form of the disease occurs at birth, which can present with absence of bone mineralization in utero, resulting in stillbirth.
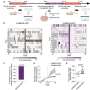
Using a mouse model, José Luis Millán, Ph.D. tested the hypothesis that, when administered from birth, a bone-targeted form of the TNAP gene would ease the skeletal defects of HPP. The Millán laboratory, in collaboration with scientists from Enobia Pharma in Montreal, Canada and from the Shriners Hospitals for Children in St. Louis, Missouri, created a soluble form of human TNAP that had been shown to display a strong attraction to bone tissue. Upon injecting the enzyme into the fat layer under the skin of the mice, the treated mice maintained a healthy rate of growth and apparent well being, as well as normal bone mineral density (BMD) of the skull, femur and spine. In fact, complete preservation of skeletal and dental structures were observed after 15 days, and bone lesions were still not seen after 52 days of treatment.
"While the biochemical mechanism that leads to skeletal and dental defects of HPP is now generally understood," said Dr. Millán, "there is currently no established medical treatment."
Given the success of this therapy in preventing HPP, current efforts in Dr. Millán's laboratory are focused on reversing the bone defects in mice once the disease is quite advanced. Future clinical trials may reveal this as the first promising therapy for patients with this genetic disorder.
This study was published in the Journal of Bone and Mineral Research.
Source: Burnham Institute