Study offers novel insight into cardiac arrhythmias, sudden cardiac death
A new study by researchers at Rhode Island Hospital provides much-needed insight into the molecular mechanisms that cause arrythmia, or irregular heartbeat, and how it triggers sudden cardiac death, one of the nation’s leading killers. Their findings, published online in the Journal of Clinical Investigation, could pave the way for the development of new, genetically-targeted therapies to treat and prevent fatal arrythmias.
Most cases of sudden cardiac death are related to arrhythmias, including clinical conditions such as long QT syndrome (LQTS), a disorder of the heart’s electrical system that causes fast, chaotic heartbeats. LQTS – which can be inherited or brought on by certain medications – usually affects otherwise healthy children and young adults. Although LQTS seems to be relatively rare, experts believe it is also underdiagnosed, meaning variants of it may be more common than previously suspected.
LQTS gets its name from a markedly elongated QT interval on an electrocardiogram (ECG). The QT interval corresponds to the time it takes for the heart’s lower chambers, or ventricles, to discharge (depolarize) and recharge (repolarize) so that they are ready for a new contraction cycle again. The problem in most patients stems from defects in the tiny channels that allow electrically charged ions, such as potassium, to flow out of the heart’s cells. This flow of ions is crucial to the proper repolarization of the heart muscle. The lengthening of the QT interval is associated with increased likelihood of triggering irregular and sometimes life-threatening arrhythmia.
“We are still struggling to understand why arrhythmia causes sudden cardiac death in some patients, but not others, and what underlying molecular mechanisms or abnormalities may be at play,” says senior author Gideon Koren, M.D., director of the cardiovascular research center at Rhode Island Hospital and a professor of medicine at The Warren Alpert Medical School of Brown University. “One of the reasons we know so little about it is that, until now, there has not been a good animal model for study.”
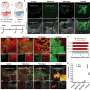
To address this critical issue, Koren and his team developed an animal model of LQTS that allows researchers to study the various mechanisms that trigger arrhythmia and cause sudden cardiac death. This is the first genetic model in the world to mimic what happens during arrhythmia in humans.
Specifically, the researchers engineered transgenic models of the two most common genetic forms of LQTS in humans – LQT1 and LQT2. In both cases, faulty genes result in the production of abnormal ion channels – the proteins responsible for moving potassium in and out of heart cells so they can contract. In LQT1, the mutation is in the KvLQT1 gene; in LQT2, the HERG gene is affected.
Scientists discovered that both models selectively eliminated their respective potassium ion channels, leading to a significant prolongation of the QT interval. However, this had two very different effects. Although the LQT1 group exhibited QT prolongation, they did not experience spontaneous arrhythmias or sudden death. In contrast, the LQT2 group, which had a more pronounced QT prolongation, had the opposite reaction – they exhibited both spontaneous arrhythmias and some of the animals died suddenly.
After using fluorescence imaging techniques to analyze the models, the team believes that the electrical cause for the deadly arrhythmias in the LQT2 group is increased spatial dispersion of repolarization across the anterior (front) of the outside layer of cardiac muscle. In contrast, the LQT1 group did not have increased dispersion, despite a similar degree of prolongation of repolarization.
A third conclusion from this study involves an apparent relationship between HERG and KvLQT1 that suggests they might interact (either directly or indirectly) and that a mutation of either of these channels could adversely affect the function of the other currents. Koren believes that these phenomena signify the importance of such animal models in understanding more about arrhythmias and sudden cardiac death.
“While results from animal models are not always applicable to humans, we believe our findings are a first steps toward gaining a better understanding of how and why arrhythmias cause sudden cardiac death. However, there is much more that we still don’t know,” he says.
Koren adds that the LQT2 model could play a major role in identifying mechanisms of sudden cardiac death, while the LQT1 model could help screen for and detect those drugs that interact with the HERG potassium channel, causing QT prolongation and potentially fatal arrhythmias.
Screening for such drugs, including certain antibiotics, could be critical for pre-menopausal women who generally have slightly prolonged QT intervals and are more sensitive to the effects of these particular medications.
Source: Lifespan