Atypical protein kinase C stabilizes SRC-3 levels in breast cancer cells
A new study provides valuable insight into a previously undescribed mechanism that regulates a prominent cancer-associated protein. The research, published by Cell Press in the February 29th issue of Molecular Cell, will enhance understanding of the fundamental processes that contribute to breast cancer.
It is well established that steroid receptor coactivator-3 (SRC-3/AIB1) plays key roles in cell growth, reproduction, metabolism and cytokine signaling, is overexpressed in many cancers and is a major player in tumorigenesis and cancer progression. It is also clear that protein kinases are often overactive in cancers and that distinct patterns of phosphorylation, induced by different signals and different kinases, can play a major role in regulating cancer-associated proteins, including SRC-3.
“Recently, it was shown that phosphorylation of SRC-3 by specific kinases is associated with increased degradation of SRC-3. However, kinases that stabilize SRC-3 in cancer cells have not yet been reported,” explains lead author Dr. Bert O’Malley from the Baylor College of Medicine. Dr. O’Malley and colleagues examined the interaction between atypical protein kinase C (aPKC), which is overexpressed in many cancers, and SRC-3.
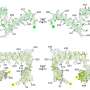
The researchers found that aPKC stabilized cellular SRC-3 protein levels by inducing phosphorylation of a particular region of SRC-3. Specifically, phosphorylation of C-terminal residues induced a conformational change that made SRC-3 more resistant to degradation by the core proteasome. This effect of aPKC required estrogen and estrogen receptor and was not supported by other steroid receptors, suggesting that aPKC-mediated SRC-3 stabilization is a receptor-selective event. These results reveal a mechanism that links aPKC with estrogen-dependent growth and tumorigenesis and provide yet another layer of control for regulating levels of the SRC-3 oncogenic protein.
“Our data describe a new regulatory mechanism for SRC-3 protein turnover which may play an important role in regulating SRC-3 levels in normal and oncogenic cell growth,” offers Dr. O’Malley. “We propose that when aPKC is overexpressed in cancer cells, the consequence is increased SRC-3 function and powerful enhancement of estrogen-receptor target gene transcription and promotion of estrogen-dependent cell growth in cancer cells such as breast.”
Source: Cell Press