Study identifies another strategy for normalizing tumor blood supply
Manipulating levels of nitric oxide (NO), a gas involved in many biological processes, may improve the disorganized network of blood vessels supplying tumors, potentially improving the effectiveness of radiation and chemotherapy.
In an upcoming issue of the journal Nature Medicine, researchers from the Steele Laboratory of Radiation Oncology at Massachusetts General Hospital (MGH) report an experiment in which NO production was selectively suppressed in tumor cells while being maintained in blood vessels. The result was a significant improvement in the appearance and function of the tumor’s blood supply.
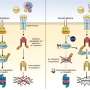
“Our finding suggest that the creation of perivascular NO gradients – differences between the levels produced in blood vessels and those found in tumor tissue – may be able to normalize tumor vasculature,” says Dai Fukumura, MD, PhD, of the Steele Laboratory, who led the study. “Combining the use of angiogenesis inhibitors, which normalize vasculature through a different mechanism, with the blockade of nonvascular NO production may produce even greater improvement in therapeutic outcomes.”
The blood vessels that develop around and within tumors are leaky and disorganized, interfering with delivery of chemotherapy drugs and with radiation treatment, which requires an adequate oxygen supply. Combining angiogenesis inhibitors, drugs that suppress the growth of blood vessels, with traditional anticancer therapies has improved patient survival in some tumors. That success supports a theory developed by Rakesh K. Jain, PhD, director of the Steele Laboratory, that the agents temporarily ‘normalize’ blood vessels, creating a period during which other treatments can be more effective.
Since angiogenesis is one of many physiologic activities mediated by NO, the MGH research team hypothesized that restricting NO production to blood vessels also could improve tumor vasculature. Using cancer cells from human brain tumors, they suppressed the enzyme that controls NO production in nonvascular tissue. When the modified tumor cells were implanted into mice, analysis of the resulting tumors showed that NO was present primarily in blood vessels, with significant reductions in tumor cells. Vessels in the growing tumors were more evenly distributed and less distorted than those in tumors grown from untreated tissue.
“Angiogenesis inhibitors block formation of new vessels by directly or indirectly inhibiting the proliferation and survival of vascular endothelial cells. But since their overall effect is to reduce the density of blood vessels, the ability of those agents to normalize tumor vasculature may not last long,” says Fukumura. “Blocking nonvascular NO production and maintaining NO levels around the vessels appears to keep endothelial cell function at the proper level.” An associate professor of Radiation Oncology at Harvard Medical School, Fukumura notes that the strategy now should be investigated in other types of tumors.
Source: Massachusetts General Hospital