String of Fullerene Pearls
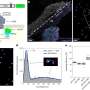
Under an atomic force microscope, the tiny structures look like fragments of nanoscopic pearl necklaces. In reality, the “pearls” are fullerene molecules that are linked together by means of a special fullerene-binding molecule. Spanish researchers describe their method for “threading” these nanopearls in the latest issue of the journal Angewandte Chemie.
Fullerenes are spherical, cage-like molecules made of 60 carbon atoms whose linkages resemble the five- and six-sided leather patches on a soccer ball. Recently, a team in Madrid headed by Nazario Martín developed a novel electroactive “fullerene receptor” molecule, a molecule that specifically recognizes and binds to the surfaces of fullerenes.
Now the researchers have gone a step further: They have produced molecular chimeras by binding their fullerene receptors to a fullerene molecule. The receptor portion is a system of eleven rings. It recognizes the fullerene portion of neighboring fullerene-receptor chimeras and grasps it from two sides like a pincer. This results in linear aggregates of molecules lined up like pearls. The researchers found fragments containing up to 35 “pearls” under the atomic force microscope.
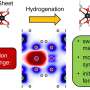
When the pincer-like receptor “grasps” the fullerene, its flat aromatic rings spread over the equally flat ring systems on the fullerene surface. This results in special binding interactions between the electrons of these ring systems. Under certain conditions, it is possible to transfer electrons between such “complementary” electron systems. This property could make these fragments interesting as a new starting material for more efficient optoelectronic components.
In any case, the formation of this supramolecular polymer represents a new approach to the controlled organization of electroactive materials.
Citation: Nazario Martín, Self-Organization of Electroactive Materials: A Head-to-Tail Donor–Acceptor Supramolecular Polymer, Angewandte Chemie International Edition, doi: 10.1002/anie.200703049
Source: Angewandte Chemie