Buckyball birth observed by Sandia nanotech researcher

Almost everyone in the scientific community has heard of buckyballs, but no one until Sandia’s Jianyu Huang has seen one being born.
Buckyballs — more formally known as buckminsterfullerene C-60 — are carbon-linked nanostructures named for their resemblance to the geodesic dome macrostructures favored for their strength by environmentalist Buckminster Fuller.
In addition to the strength generated by their carbon-carbon bonds — “the strongest chemical bonds in Mother Nature,” says Huang, who still seems awed by the properties of the nanomaterial — the structure forms a relatively impermeable cage that conceivably could safely transport molecules of hydrogen for fuel, or tiny doses of medicine to targeted sites within the human body.
But before their widespread use is possible, buckyballs have to be available in large numbers. To achieve that, better understanding of how they form is crucial.
“We have now the first direct, in situ, experimental proof of the hypothesis — very significant to the scientific community — that these structures are formed by the heated ‘shrink-wrapping’ of carbon sheets,” says Huang.
That is, heating bends single-atomic-layer carbon sheets into nano bowls, and then adds more carbon atoms to the edge of the bowls until the formation of giant fullerenes — larger, less stable versions of the C-60 molecule. Continued application of heat reduces these fullerenes — “shrink-wrapping” is the favored term — to the size of stable C-60 molecules, the buckyball: the smallest stable arrangement of carbon atoms in that shape.
In further heating, the buckyball vanishes, providing more proof that the buckyball stage had been reached.
Buckyball codiscoverer (1985) and Nobel laureate (1996) Richard Smalley had hypothesized that buckyballs are formed in this fashion, but at his death in 2005 no experimental confirmation was yet available and other methods have been proposed.
A paper detailing the work was published in the Oct. 26 Physical Review Letters.
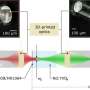
Huang’s discovery happened unexpectedly. He was in fact looking for flaws in nanotube durability. Transmitting electric current through the atom-sized tip of a scanning tunneling microscope (STM) — itself inside a transmission electron microscope (TEM) — he had heated a 10-nanometer-diameter multiwalled carbon nanotube to approximately 2,000 degrees Celsius when he saw the exterior shells of giant fullerenes form from peelings within the nanotube. High-resolution 2-D images of the process taken by a CCD camera attached to the microscope showed the fullerenes reducing in diameter, linearly with time, until the structures became the size of C-60, the smallest arrangement of carbon atoms that form the soccerball shape.
Then the buckyballs vanished.
Simulations created at Huang’s request by Boris Yakobson’s team at Rice University, who coauthored the Physical Review paper, show that heating could reduce fullerenes by emitting carbon dimers (pairs of atoms) until they reached the basic buckeyball shape. Further removal of carbon pairs collapsed the structure.
Buckyballs are formed by hexagonal and pentagonal arrangements of carbon atoms that seem stitched or welded together, in appearance much like a soccer ball. Their curvature, however, is caused by the pentagons alone, 12 to a buckyball. Departing atoms leave the same number of pentagons until the fullerene shrinks below its smallest stable shape, below which the buckyball disintegrates.
“I used to study metals,” says Huang, who grew up in a remote Chinese farming village and now utilizes the most complex instruments at Sandia’s Center for Integrated Nanotechnologies (CINT). “But carbon nanomaterials now are much more interesting to me.”
CINT is a joint effort of Sandia and Los Alamos national labs and is supported by the DOE’s Office of Science.
The buckyball discovery was initially made by Huang on similar instruments at Boston College, and then interpreted at CINT.
“The STM probe inside the TEM is a very powerful tool in nanotechnology,” Huang says. “The STM probe is like God’s finger: it can grab extremely small objects, as small as a single atomic chain, enabling me to do nanomechanics, nanoelectronics, and even thermal studies of carbon nanotubes and nanowires.”
Watch Movie: Fullerene Sublimation -- www.sandia.gov/videos2007/2007 … ereneSublimation.mpg
Source: Sandia National Laboratories