Cancer drug works by overactivating cancer gene
University of Michigan Comprehensive Cancer Center researchers have discovered that bortezomib, a promising cancer drug, is able to strike a blow against melanoma tumor cells by revving up the action of a cancer-promoting gene.
They say the laboratory-based findings suggest a novel treatment strategy that might someday prove effective against many types of cancer: Push cancer cells into overdrive, so that they self-destruct.
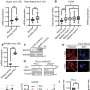
The U-M scientists found that bortezomib, a drug approved by the FDA to treat advanced multiple myeloma, is able to selectively inhibit melanoma tumor cells because it causes the c-MYC oncogene to overproduce a cell-death promoter called NOXA. Their results place c-MYC and NOXA, well studied among cancer researchers, in a new light. The study appears online ahead of print in the Proceedings of the National Academy of Sciences.
“Our data suggest a different approach to treat cancer,” says Maria S. Soengas, Ph.D., the senior author of the study. Soengas is an assistant professor of dermatology at the U-M Medical School and a member of the U-M Comprehensive Cancer Center.
Many cancer treatments aim to block specific oncogenes, genes that wreak havoc with the normal signals that dictate when cells multiply and die.
The thinking is that if oncogenes are disabled, cancer cells can’t proliferate uncontrollably and spread. However, scientists know that oncogenes can play dual roles: They can cause tumor cells to rapidly divide, but can also step up programmed cell death, or apoptosis.
Therefore, “an alternative treatment could be to actually exacerbate oncogene function, to promote such a dysregulation of cell cycle progression and activation of apoptotic proteins that tumor cells ultimately die,” says Soengas.
Melanoma tumor cells manage to resist most cancer drugs. For more than 30 years, the prognosis for patients with advanced melanoma has not significantly improved. Soengas likens the melanoma tumor cell’s defenses to a heavy suit of armor that so far has blocked researchers’ attempts to penetrate it. Now it appears that the tumor cells have an enemy within.
In human melanoma cells cultured and manipulated in the laboratory, Soengas and her team have studied bortezomib and other drug candidates to understand their molecular modes of action.
Bortezomib belongs to a class of drugs called proteasome inhibitors that show promise in attacking many types of tumors. But how the drugs direct their biggest punch at tumor cells, with less effect on normal ones, has puzzled scientists – the cell actors they target, proteasomes, are widespread and essential to normal cells.
Soengas and colleagues reported in 2005 that bortezomib appears to combat tumor growth by increasing the activity of a cell-death promoter called NOXA in tumor cells, but not in normal cells. In the new study, the U-M scientists report that the force behind this selective uptick in NOXA, and the resulting cell death, surprisingly turned out to be the oncogene c-MYC.
The discovery of the oncogene’s role in bortezomib’s action has implications for other cancers besides melanoma, says Mikhail Nikiforov, Ph.D., the paper’s first author. The Soengas and Nikiforov groups collaborated to elucidate molecular mechanisms of c-MYC-mediated regulation of NOXA in melanoma and other tumor cell types. Nikiforov is an assistant professor of dermatology at the U-M Medical School and a member of the U-M Comprehensive Cancer Center.
The findings lay the groundwork for more studies to improve bortezomib’s effectiveness in treating cancers and to reduce its toxicity in normal cells, Soengas says.
“Now we can rationally design drugs that enhance bortezomib’s action and favor NOXA production,” she says. “Improvements might make it possible to give lower doses of the drug for a shorter time.”
These improvements to bortezomib treatment, as well as other drugs that could take advantage of the study results, will take years of testing before they can possibly help patients. Soengas and her colleagues are collaborating with other U-M scientists on several projects, including one led by Shaomeng Wang, Ph.D., associate professor of hematology/oncology and pharmacology, to design drugs that will favor the effects of NOXA.
Clinical trials to test bortezomib’s effects on other types of tumors are under way at the U-M and around the country.
Source: University of Michigan