Improved Electrodes: Electrodeposition Process Creates Nanoporous Structures for Improved Fuel Cells, Batteries & Sensor
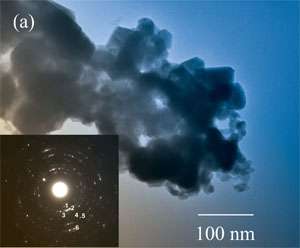
Taking advantage of an electrochemical phenomenon that had previously been considered a nuisance, researchers at the Georgia Institute of Technology have developed a new class of three-dimensional nanoporous electrodes that could boost the performance of fuel cells, batteries and sensors.By generating hydrogen bubbles during the deposition of copper, tin or a copper-tin alloy onto a copper substrate, the researchers create self-supported metallic foam electrodes that contain a complex network of interconnected pores. Because the bubbles expand as they move away from the substrate, they create passageways through the deposited metal that become wider the closer they get to the outside of the electrode.
The tapered passageways should allow gases and fluids to move more easily through these “functionally-graded” electrodes, boosting the performance of solid-oxide fuel cells, lithium batteries and chemical sensors. The nanoporous nature of the structures provides a large surface area on which electrochemical reactions can take place.
“By adjusting the properties of the electrolyte – the viscosity and chemical composition – we can change the size of the gas bubbles we generate,” explained Meilin Liu, a professor in Georgia Tech’s School of Materials Science & Engineering. “Getting the bubbles small enough allows us to produce three-dimensional nanostructures in which the pores are small on the inside but taper to larger pores on the outside.”
The research, supported by the Office of Basic Energy Sciences in the U.S. Department of Energy, has been reported in the journal Advanced Materials. An additional paper describing fabrication of copper-tin alloy electrodes and their application to lithium batteries has been accepted for publication in Advanced Functional Materials.
Existing techniques for producing functionally-graded electrodes require the deposition of multiple layers of material, each with a different pore structure. Each layer must be thermally and chemically compatible and able to conduct electricity. Therefore, the process developed by Liu and collaborators Heon-Cheol Shin and Jian Dong offers a simpler means of producing electrodes that facilitate the movement of liquids and gases.
“In our electrode, the gradient is created naturally and is ideal for our needs,” explained Liu, co-director of Georgia Tech’s Center for Innovative Fuel Cell and Battery Technologies. “That is really the utility of this process. You can avoid the complexity of creating multiple layers.”
Previous efforts to create metallic foams have been plagued by structural weaknesses in which the passageways carved into the electrodes cause them to collapse. Liu and his team have solved that problem by carefully controlling the competitive reaction rates for gas evolution and metal deposition, forming dendritic branches that are mechanically well-supported and stable.
Production of hydrogen bubbles serves as the basic sculpting tool for creating the pore structure. The gas acts as a dynamic template for the formation of the structure, and serves as a barrier for the diffusion of reactive ions from the electrolyte to regions around the branches that are depleted of ions, preventing overgrowth of passageways.
Hydrogen production is normally undesirable in electrochemical deposition processes because it can reduce the density of the resulting metal films.
The new electrodes vary in thickness from a few microns up to 15 microns, depending on the materials used and the processing time. Liu expects that copper-based electrodes will be useful in solid oxide fuel cells, while tin-based electrodes will be useful in lithium batteries.
Microscope study reveals subtle differences between electrodes made from copper and those made from tin. The branches of the tin deposits are longer and straighter than those of copper. The copper foams also contain nanometer-sized grains and pores not seen in the tin structures.
Liu believes the differences may be related to the fact that during deposition of the copper electrodes, gas bubbles are produced from both the deposited copper and the substrate. In the tin electrodes, only the substrate produces bubbles.
While the new electrodes have not yet been tested in fuel cells or batteries, Liu expects they will significantly boost energy output.
“These electrodes will significantly reduce the resistance to mass transport because gas can flow in easily,” he said. “The nanostructure also provides a very high surface area, so the charge transfer can occur more easily. As a result, the overall resistance of the electrode will be reduced, and the energy production increased.”
So far, the researchers have produced only small electrode samples, typically about two square centimeters in size. Liu sees no reason why the process could not be scaled up to produce larger electrodes, but for now is focusing on better understanding process parameters that affect the resulting materials.
“We are trying to understand the process and how we can control and tailor the nanostructure by changing the process conditions,” Liu explained. “Once we have a full understanding of how to control the structure, the next step will be to make the electrode for actual applications in batteries and fuel cells.”
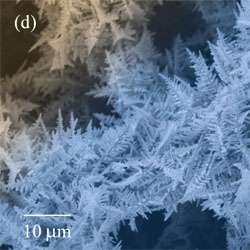
For the future, the researchers also plan to study whether the process can be used to fabricate nanoporous electrodes from other metals, metal oxides and alloys.
A specialist in high-temperature solid-oxide fuel cells, Liu has been studying potential efficiency improvements for many years. The new process resulted from continuing efforts to improve the efficiency of both fuel cells and batteries.