Implantable device designed to detect, stop seizures under study

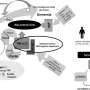
A small device implanted in the skull that detects oncoming seizures, then delivers a brief electrical stimulus to the brain to stop them is under study at the Medical College of Georgia.
MCG is among 28 U.S. centers participating in a study to determine if the neurostimulator device can help patients whose seizures are not well controlled by drugs.
“The device constantly monitors electrical activity of the brain, gets accustomed to what is normal for that patient and, when it detects activity that is abnormal, within a few milliseconds, sends out a small electrical stimulus to stop it,” says Dr. Yong Park, MCG pediatric epileptologist and a principal investigator.
At MCG Medical Center, the RNS System, developed by California-based medical device manufacturer NeuroPace, will be used in about 10 patients age 18-70 who have failed to get their seizures controlled with at least two medications. About 240 patients are expected to enroll nationwide.
Eligible participants must have at least three seizures per month and no more than two seizure foci in the brain. Seizure activity is closely monitored through a diary and monthly doctor visits for three months before patients become eligible.
Participants have a device implanted in the skull, with up to two wires containing electrodes placed near the seizure focus. A modified laptop computer looks at electrical activity picked up by the neurostimulator, then is used to program the device to recognize a patient’s seizure activity. Physicians can continue to fine-tune the detection and stimulation patterns.
During the first month after implant, the RNS™ is set for detection only while doctors design a set of parameters that allow it to reliably detect the onset of seizure activity, says Dr. Patty Ray, study coordinator. After one month, half of the patients are set for detection and responsive stimulation, the other half continue with detection only. After four months, all devices are set for detection and responsive stimulation throughout the remainder of the two-year study. After the study, patients will be eligible for a study to continue to use the device until it receives FDA approval, Dr. Ray says.
Participants remain on anti-seizure medication throughout the study, although Dr. Park suspects some patients may eventually be weaned off drugs. During this study, researchers also are collecting data on which drugs work best with the neurostimulator.
MCG participated in a smaller feasibility study of the neurostimulator in 2004 and prior to that was among the first centers in the country to use it as an external, temporary measure to try to stop seizures in hospitalized patients whose seizure activity was being monitored, Dr. Park says.
About 1 in 200 people have seizures and about 1 out of 3 cannot get seizures under control with one or more medications. Some people are not candidates for traditional epilepsy surgery to remove the seizure focus because the location increases the risk of problems or deficits, because there are too many foci or because they simply do not want the surgery, Dr. Park says. Early evidence indicates the RNS™ device might be most effective for foci in the medial temporal lobe, an area deep inside the brain involved in memory, where surgery is not an option, he says.
A handful of new drugs, with generally fewer side effects, also have become available in the last few years, giving patients and their doctors more options, he says. MCG has begun studies of three additional anti-seizure medications.
Also on the horizon is a mechanism that delivers a bolus of drug directly to the seizure focus in the brain, which may enhance efficacy and decrease side effects, as well as gene therapy to modify abnormal brain tissue, says Dr. Park, who wants MCG to participate when those studies move to clinical trials.
Source: Medical College of Georgia