McGill study promises faster-acting anti-depressants
A McGill University study has found that a new class of drugs known as serotonin4 (5-HT4) receptor agonists may take effect four to seven times faster than traditional selective serotonin reuptake inhibitors (SSRIs). The study, led by former McGill post-doctoral fellow in psychiatry Guillaume Lucas with his supervisor, the late Dr. Guy Debonnel, was published in the September 6 issue of the journal Neuron.
Existing SSRI-class drugs, widely prescribed as anti-depressants, can take up to six weeks to become effective, with potentially serious clinical consequences. Dr. Lucas, now an associate researcher at the Centre de Recherche Fernand Séguin of Université de Montréal, said, "These delays are not only a matter of patient comfort, it's really important, especially when you are treating major depressions that could lead to suicide."
SSRIs work by enhancing the available concentration of the neurotransmitter serotonin in the brain. The McGill study focused on a new class of drugs known as serotonin4 (5-HT4) receptor agonists, which act directly on the nerve impulses of serotonin neurons.
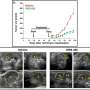
In behavioral tests, rats on two different serotonin4 receptor agonists showed marked improvements in symptoms of chronic depression after only three days and were symptom-free after a week. In subsequent tests, three days of treatment with serotonin4 receptor agonists induced anti-depressant-related effects in the brains of the animals seen only after weeks of treatments with SSRIs.
Source: McGill University