Atomic layer deposition fuels future solutions to nation's energy challenges
More efficient and less costly solar cells, solid-state lighting and industrial catalysts are potential applications of atomic layer deposition (ALD), a technique that researchers at Argonne National Laboratory are working to perfect. Other potential applications are improved superconductors and separation membranes.
ALD is a thin-film growth technique that offers the unique capability to coat complex, three-dimensional objects with precisely fitted layers. The scientists expose an object to a sequence of reactive gas pulses to apply a film coating over the object's surface. The chemical reactions between the gases and the surface naturally terminate after the completion of a "monolayer" exactly one molecule thick. ALD can deposit a variety of materials, including oxides, nitrides, sulfides and metals.
What makes ALD more effective and flexible than traditional methods for producing thin film coatings, such as evaporation, is its ability to coat every nook and cranny of a complex object.
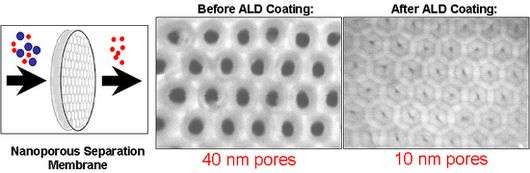
Scientists use this procedure to fabricate nanostructured catalytic membranes, or NCMs. These structures enable catalytic reactions that, for example, convert inexpensive feedstocks into valuable products and synthesize hydrocarbon fuels. Argonne has filed for a patent on NCMs.
“We are focusing our attention now on measuring the properties of the catalysts and synthesizing other catalytically relevant materials inside the NCMs,” said Jeffrey Elam, a research chemist in Argonne's Energy Systems Division.
Elam, along with Michael Pellin of Argonne's Materials Science Division, has been working with NCMs to carry out chemical reactions to produce materials that help the nation sustain itself in a more cost-effective and efficient manner.
One of the Argonne researchers' goals has been to improve the effectiveness of the catalyst in Fischer-Tropsch synthesis. The Fischer-Tropsch process takes syngas, a mixture of carbon monoxide and hydrogen, and converts it into hydrocarbon fuels. Syngas can come from a variety of materials, including natural gas, coal or biomass.
Elam and Pellin hope that Argonne's NCMs can improve the performance of Fischer-Tropsch catalysts enough to make the production of clean, sulfur-free fuels economically viable in the next decade or two.
Recently, Argonne researchers also have begun to apply ALD technology to solid-state lighting, which uses light-emitting diodes, or LEDs. Unlike incandescent light bulbs, LEDs consume little electric power and do not burn out or overheat. They are illuminated by the movement of electrons in a semiconductor and are considered the most efficient light source in existence. LEDs can be found in many electronic devices, from digital displays to traffic lights.
LEDs require a conducting electrode to supply electricity to the semiconducting material, but this electrode must also be transparent to allow the light to escape. Traditionally, this transparent conducting electrode is made from indium-tin oxide (ITO); however, ITO is too expensive for mass production.
To replace ITO, Argonne researchers are exploring chains of metal nanoparticles aligned in a magnetic field to form an electrically conductive web. ALD coatings are applied to these networks to form a transparent, conducting electrode to make cheaper LEDs. This research is funded by the U.S. Department of Energy to develop advanced solid-state lighting technologies that, compared to conventional lighting technologies, are much more energy efficient, longer lasting and cost-competitive by 2025.
In cooperation with Northwestern University, Argonne researchers are also fabricating highly efficient solar cells for converting sunlight into electricity. These improved, dye-sensitized solar cells (DSSCs) use ALD technology in a similar way to NCMs – precisely fitted layers of transparent, conducting oxides and semiconductors are deposited on the inner surfaces of nanoporous membranes.
The researchers aim to eventually commercialize these novel and efficient solar cells. Because no pure, costly silicon is involved in the fabrication process—as it generally is with conventional solar cells—the researchers hope to produce electricity at a much lower cost.
Source: Argonne National Laboratory