Measuring the unseeable: Researchers probe proteins' 'dark energy'
Researchers at the University of Pennsylvania School of Medicine are the first to observe and measure the internal motion inside proteins, or its “dark energy.” This research, appearing in the current issue of Nature has revealed how the internal motion of proteins affects their function and overturns the standard view of protein structure-function relationships, suggesting why rational drug design has been so difficult.
The situation is akin to the discussion in astrophysics in which theoreticians predict that there is dark matter, or energy, that no one has yet seen,” says senior author A. Joshua Wand, PhD, Benjamin Rush Professor of Biochemistry. “Biological theoreticians have been kicking around the idea that proteins have energy represented by internal motion, but no one can see it. We figured out how to see it and have begun to quantify the so-called ‘dark energy’ of proteins.”
Proteins are malleable in shape and internal structure, which enables them to twist and turn to bind with other proteins. “The motions that we are looking at are very small, but very fast, on the time scale of billions of movements per second,” explains Wand. “Proteins just twitch and shake.” The internal motion represents a type of energy called entropy.
Current models of protein structure and function used in research and drug design often do not account for their non-static nature. “The traditional model is almost a composite of all the different conformations a protein could take” says Wand.
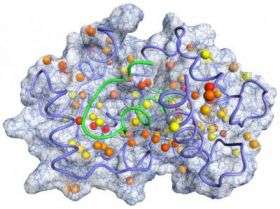
The researchers measured a protein called calmodulin and its interactions with six other proteins when bound to a protein partner one at a time. These binding partners included proteins important in smooth muscle contraction and a variety of brain functions.
Using nuclear magnetic resonance spectroscopy, the investigators were able to look at the changes in the internal motion of calmodulin itself in each of the six different protein binding situations. They found a direct correlation between a change in calmodulin’s entropy –a component of its stored energy – and the total entropy change leading to the formation of the calmodulin-protein complex. Finding out the contribution from individual proteins versus the entropy, or movement, of the entire protein complex has been more difficult and has been overcome in this study. From this individual contribution they deduced that changes in the entropy of the protein are indeed important to the process of calmodulin binding its partners.
“Before these unexpected results, most researchers in our field would have predicted that entropy’s contribution to protein-protein interactions would be zero or negligible,” says Wand. “But now it’s clearly an important component of the total energy in protein binding.”
Because of this new information, the researchers suggest that the entropy component may explain why drug design fails more often than it works. Currently, drugs are designed generally based on the precise structures of their biological targets, active regions on proteins that are intended to inhibit key molecules. However, the number of designed molecules actually binding to their targets is low for many engineered molecules. “We think that this is because the design is based on a model of a static protein, not the moving, hyper protein that is constantly changing shape,” say Wand. “We need to figure out how this new information fits in and perhaps drug design could be significantly improved.”
Future directions include understanding whether the principles revealed by this study are universal and impact the thousands of protein-protein interactions that underlie biology and disease. As Wand explains, “Protein-protein interactions are central to ‘signalling’, which is often the molecular origin of diseases. Cancer, diabetes, and asthma are three important examples. We are currently looking at the role of protein entropy in the control of critical signaling events in all three.”
Source: University of Pennsylvania