Gut check: Tracking the ecosystem within us
For more than 100 years, scientists have known that humans carry a rich ecosystem within their intestines. An astonishing number and variety of microbes, including as many as 400 species of bacteria, help humans digest food, mitigate disease, regulate fat storage, and even promote the formation of blood vessels. By applying sophisticated genetic analysis to samples of a year’s worth baby poop, Howard Hughes Medical Institute researchers have now developed a detailed picture of how these bacteria come and go in the intestinal tract during a child’s first year of life.
The study, published June 25, 2007, in the journal Public Library of Science (PLoS) Biology, was led by Howard Hughes Medical Institute (HHMI) investigator Patrick O. Brown at the Stanford University School of Medicine.
"I don't know what a human would look like without a colonized gut," said Chana Palmer, the lead author of the new study and a former graduate student in Brown's lab. "The microbiota are important. They help you extract more from your food; they're important for the immune system; and they help protect us from being colonized by [microbes] that are going to do us harm."
Before birth, the human intestinal tract is sterile, but babies immediately begin to acquire the microbial denizens of the gut from their environment -- the birth canal, mothers' breast, and even the touch of a sibling or parent. Within days, a thriving microbial community is established and by adulthood, the human body typically has as many as ten times more microbial cells than human cells. This is primarily due to the large number of microorganisms that have taken up residence in the intestine.
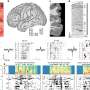
The new study tracked the evolution of the microbial ecosystems in 14 healthy, full-term human infants that were breast fed. Most of the bacteria that live within humans do not thrive in an oxygen-rich environment, and thus are difficult or impossible to grow in culture in the lab. So the researchers turned to DNA microarray technology. That technology, developed by Brown in the 1990s, allows researchers to simultaneously measure the presence or activity of thousands of genes. The team used microarrays to profile the mixture of bacterial DNA in an average of 26 stool samples per infant over the course of the first year of life, beginning with the first stool after birth.
For a handful of these samples, they compared the results that they had obtained using their microarray with the laborious ‘gold standard’ approach of using genetic libraries of bacteria to get a snapshot of the microbial ecosystem in an infant at a given point in time and found that their new method performed very well.
The results, said Palmer, were striking: the group found that the intestinal microbial communities varied widely from baby to baby – both in terms of which microbes were present and in how that composition changed over time. That finding, she said, is important because it helps broaden the definition of healthy microbial colonization in a baby.
Another intriguing observation, Palmer noted, was a tendency for sudden shifts in the composition of the infants' intestinal microbial communities over time as different species of bacteria ebbed and flowed.
"We don't have a good explanation for why one big group of bacteria would replace another. And it's not that the number of bacteria dropped," Palmer explained. "The size of the population was relatively stable."
Over time, however, the composition of the intestinal microbial communities converged toward a more generic profile characteristic of the adult intestine.
The new study, the authors noted, might bring some clarity to the factors that shape the composition of the microbial communities in the infant intestinal tract. For example, there are conflicting studies about Bifidobacteria, a group of bacterial species reputed to have beneficial effects. Some studies have shown that it is more common in the intestinal tracts of breast-fed infants, but Palmer and Brown’s work documented a paucity of those bacteria, although all were breast fed.
The finding that most babies in the study did not acquire significant numbers of Bifidobacteria until several months after birth was a surprise, Palmer said: "That's definitely a contentious area. A lot of studies say they are a major constituent of gut flora beginning shortly after birth."
Putting the study on a firmer footing was the fortuitous inclusion of a pair of fraternal twins, Palmer noted. Although the researchers observed variability within their study, the composition and dynamics of the evolving microbial ecosystems were strikingly similar in the twins. This, Palmer said, provided evidence that genetic and environmental factors shape those ecosystems in reproducible ways. "These data are so rich it is hard to benchmark," she explained. "It's nice to have that check with the twins."
An important general conclusion, said Palmer, is that by the end of the first year of life the intestinal microbial ecosystems assumed a generally similar profile.
"It almost doesn’t matter where you start off because we all end up in the same place. There are some bacteria that are really well suited for your gut and they're going to win no matter what."
Source: Howard Hughes Medical Institute