This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cyborg technology analyzes the functional maturation of stem-cell derived heart tissue
Research in animal models has demonstrated that stem-cell derived heart tissues have promising potential for therapeutic applications to treat cardiac disease. But before such therapies are viable and safe for use in humans, scientists must first precisely understand on the cellular and molecular levels which factors are necessary for implanted stem-cell derived heart cells to properly grow and integrate in three dimensions within surrounding tissue.
New findings from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) make it possible for the first time to monitor the functional development and maturation of cardiomyocytes—the cells responsible for regulating the heartbeat through synchronized electrical signals—on the single-cell level using tissue-embedded nanoelectronic devices. The devices—which are flexible, stretchable, and can seamlessly integrate with living cells to create "cyborgs"—are reported in a Science Advances paper.
"These mesh-like nanoelectronics, designed to stretch and move with growing tissue, can continuously capture long-term activity within individual stem-cell derived cardiomyocytes of interest," says Jia Liu, co-senior author on the paper, who is an assistant professor of bioengineering at SEAS, where he leads a lab dedicated to bioelectronics.
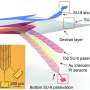
Liu's team, which specializes in engineering nanoelectronics to bridge the gap between living tissue and electronics, has developed several mesh-like, minimally invasive flexible nanoelectronic sensors designed to be embedded with natural tissues without disturbing normal cellular grown or function.
"Nature showed us the solution to monitoring tissue in 3D," Liu says. "We were inspired by the way neural tubes fold during development, stretching as cells migrate and take shape into tissue volume."
His team created their first cyborg organoid in 2019 to test the idea of using a mesh-like nanoelectronic structure, and has previously demonstrated that these types of flexible nanoelectronics can be safely implanted into living mice without disrupting the function of nearby cells.
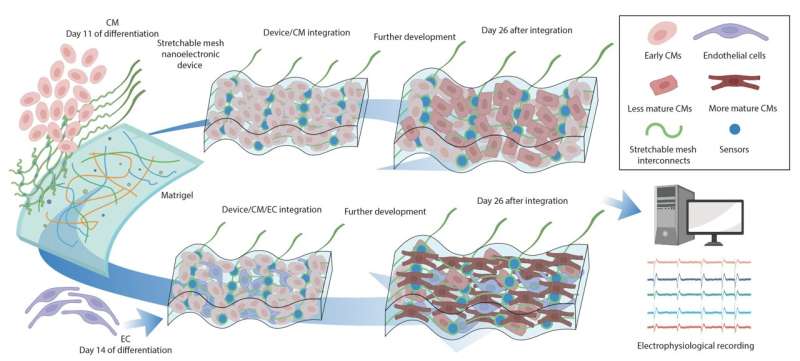
In their latest study, Liu's lab joined forces with Richard Lee and his team at Harvard Stem Cell Institute and used the nanoelectronics to monitor electrical activity of stem-cell derived cardiomyocytes. To do so, the researchers cultured cells onto a sheet made of commercially available cellular matrix known as "Matrigel" and the mesh-like nanoelectronic sensor (which contains a flexible grid of microelectrodes).
As the cells grew and developed into a small organoid structure, the researchers observed that the sheet easily stretched and accommodated the stem-cell derived tissues as they proliferated and expanded in 3D.
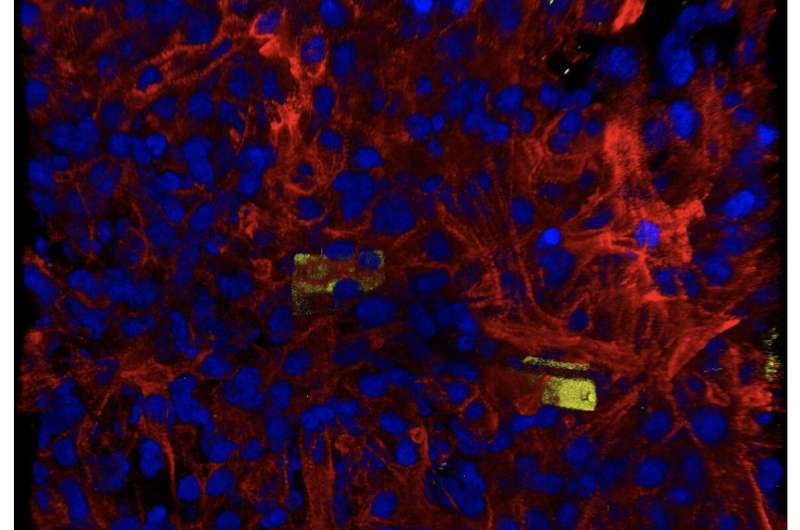
Using these techniques in in vitro experiments, the team discovered that the blood vessel lining cells that regulate blood flow between vessels and surrounding tissues (called endothelial cells) play a previously underestimated but crucial role in the rapid and functional maturation of stem-cell derived cardiomyocytes. When cultured together in a 3D cardiac tissue matrix, cardiomyocytes underwent "extraordinary electrical maturation" in the presence of endothelial cells.
Over the course of seven weeks of monitoring the developing organoids, the team observed that proximity to endothelial cells had a direct impact. Cardiomyocytes cultured next to endothelial cells matured faster compared to cardiomyocytes located farther away from endothelial cells, and they also displayed electrical characteristics typically found in healthy heart tissue.
The new insight is a leap forward for engineering stem-cell derived cardiac tissues. Experimental preclinical research in animals with human-like hearts has proven it's difficult to engineer and transplant stem-cell derived cardiomyocytes that can beat in tandem with surrounding heart tissue for extended periods of time. Immature cardiomyocytes transplanted into an animal's heart tend to beat to their own drum, and this electrical misfire can cause dangerous irregular heartbeats.
That's why the discovery that co-culturing stem-cell-derived cardiomyocytes with endothelial cells can create more functionally mature cardiomyocytes is so significant.
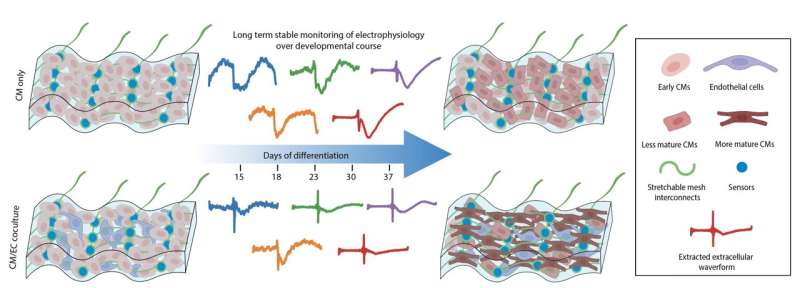
In their new paper, the team also describes using a novel machine-learning-based analysis to interpret the electrical activity captured by the tissue-embedded nanoelectronic devices, enabling continuous monitoring of the electrical waves generated by maturing cardiomyocytes of interest and enabling a better understanding of how the tissue microenvironment influences electrical stability.
Liu says the nanoelectronic devices and machine-learning-based analysis represent new platform technologies for monitoring and managing stem-cell derived tissue implants—enabling scientists to culture cyborgs made from both living tissues and electronics that can be controlled with a high degree of specificity.
In cardiac tissues, he envisions that someday these cyborgs could even be used in a sophisticated, real-time feedback system to detect abnormal electrical activity in cardiomyocytes and provide highly targeted voltage, acting like a nanoscale pacemaker, to help correct implanted cells and ensure they continue to beat in rhythm with the rest of the heart.
"If we have both nanoelectronic sensors and stimulators, we can monitor electrical activity and use feedback to pace implanted tissues into the same frequency as surrounding tissues," Liu says. "This approach could be adapted to so many other types of stem-cell-derived tissues, such as neuronal tissues and pancreatic organoids."
He also says this nanoelectronics platform approach could be used in drug screening, providing single-cell-level, continuous analysis of how tissues respond to different compounds and therapies.
More information: Zuwan Lin et al, Tissue-embedded stretchable nanoelectronics reveal endothelial cell–mediated electrical maturation of human 3D cardiac microtissues, Science Advances (2023). DOI: 10.1126/sciadv.ade8513. www.science.org/doi/10.1126/sciadv.ade8513
Journal information: Science Advances