Complex metabolism may have self-assembled from simple precursors

All modern life uses energy to reproduce itself. During this process, organisms build and break down larger molecules such as fats and sugars using a remarkably common set of reactive intermediate energy carrier molecules. These intermediate energy carriers (for example, ATP) are often not building blocks in and of themselves, but they allow the energy coupling between separate reactions needed to push forward cellular reproduction.
One class of these compounds is thioesters, chemical compounds that contain a high-energy carbon-sulfur bond. Researchers have speculated for some time that thioesters might be among the most ancient versions of such metabolic reactive intermediates, partly because modern organisms still use thioesters to break down sugars and make proteins from amino acids. Just how reactive intermediate compounds like thioesters could have developed before life evolved, or as early life was taking its first baby steps, remains shrouded in mystery.
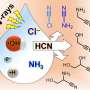
New work from researchers at the Earth-Life Science Institute (ELSI) at Tokyo Institute of Technology shows that a simple compound found in some modern volcanic gasses, a thioacid (a compound formed from an organic acid and hydrogen sulfide), readily reacts with simple sulfur-containing thiol compounds similar to those fundamental to modern metabolism to form reactive thioesters similar to those found in modern biology. This reaction happens readily in water and may have served as a starting point for the evolution of more complex biochemistry. During the research, the researchers found that when they included iron in their reactions (which is highly abundant on Earth), the reaction product yields increased.
The team suggests this may mean energy coupling between multiple reactions, in which one reaction drives another forward, may have its origins in non-living environmental chemistry. Remarkably, they also found a side product of the reaction can be used to make a second type of versatile energy-coupling compound required by all living things: FeS (short for iron-sulfur) clusters. These are small aggregates of only a few atoms each of iron and sulfur, which help organisms metabolize as they move electrons from one molecule to another. One important example of such a FeS-cluster-using-pathway is photosynthesis, which transfers electrons from water to CO2 to make sugars and oxygen. This work thus provides a new understanding of how high-energy molecules and electron transfer reactions may have been naturally produced as early metabolism was evolving.
Although scientists have been trying for some time to understand the origins of life's building blocks, little effort has been given to understanding how energy transfer originated in prebiotic chemistry. Understanding this energy exchange could be as important as understanding the origin of building blocks, so the ELSI team decided to search for reactions that could be coupled together energetically.
Lead author Sebastian Sanden says, "We were already studying FeS minerals, and we knew how easy their formation was, so we wanted to see if we could couple this excess wasted energy to another reaction." The thioacid they initially studied contains sulfur, which they knew then only had to be reacted with iron to make the FeS clusters they were already studying.

The experiments and analyses the ELSI researchers performed had to be done in rapid succession to track the progress of the reaction. They developed techniques for doing this, and thus they were able to determine how fast these reactions happened. Their preliminary thioester-making experiments did not proceed as quickly as they initially hoped, but by adding a catalyst and increasing the temperature, they found that maximum thioester yields were obtained in less than an hour, as opposed to a few days before making these changes.
The team thinks it is especially fascinating that these kinds of reactions may create "cascade reactions," which make more and more complex molecules: Pyruvate decomposes, helping form a thioester, which then allows peptides (smaller cousins of proteins) to form via the newly discovered thioester route. The team hopes to test this experimentally next and to create a system that can increase the number of components it contains by itself, perhaps all the way to self-reproduction.
In fact, some modern microbes use pyruvate decomposition and thioester formation assisted by FeS clusters in their metabolism, and it is possible that the reactions the team discovered recapitulate how early prebiological or biological evolution discovered them. Lead investigator, ELSI Associate Professor Shawn McGlynn, says, "This work provides new connections between multiple prebiotic reaction components that may have been critical to establishing early energy metabolism on Earth."
While this work may shine new light on how naturally occurring energy exchange reactions may have helped jump-start metabolism, it may also be important for the field of green chemistry, which is concerned with finding the most energetically effective and environmentally friendly methods to make chemical compounds. While toxic heavy metals like cadmium and mercury and solvents like chloroform are often used in industrial organic chemistry, the reactions discovered by this research group are very efficient and work in water using non-toxic iron as a catalyst.
More information: Sebastian A. Sanden et al, Simultaneous synthesis of thioesters and iron–sulfur clusters in water: two universal components of energy metabolism, Chemical Communications (2020). DOI: 10.1039/d0cc04078a
Journal information: Chemical Communications
Provided by Tokyo Institute of Technology