Researchers develop breakthrough process to create cancer-killing drugs
A research team at Dartmouth College has developed a new strategy for drug discovery and development that can be used to produce targeted therapies against diseases such as cancer and neurodegeneration, according to a study published in Nature Communications.
It is hoped that the process will also be useful in the large-scale production of new pharmaceuticals.
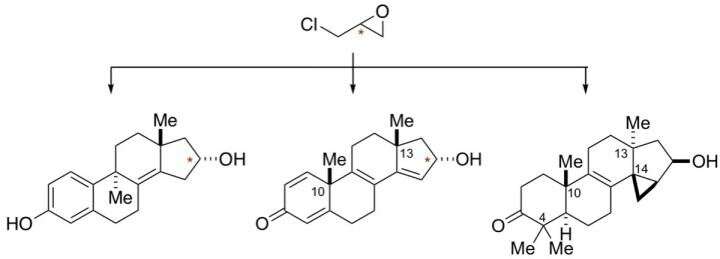
The new technique uses a novel synthesis approach for a class of organic compounds known as tetracyclic terpenoids. Tetracyclic terpenoids are responsible for more than 100 FDA-approved drugs and are considered the most successful class of natural product-inspired pharmaceuticals.
"Until now, there was nothing like this available for drug discovery and development," said Glenn Micalizio, the New Hampshire Professor of Chemistry at Dartmouth. "While additional development is expected to enhance the power of this new technology, I believe that we are at the beginning of establishing a truly enabling and potentially transformative technology for the pharmaceutical industry."
The process combines two new chemical reactions that establish bonds between carbon atoms with a unique metal-centered ring-forming reaction also developed by Micalizio.
The new technique allows for uniting molecular building blocks en route to a terpenoid skeleton in just a few chemical transformations. The result is a uniquely efficient and flexible means of enabling drug discovery in this area of natural product-focused medicinal science.
Combined, these reactions allow for exploration of pharmaceutically-privileged regions of chemical space through the straightforward conversion of inexpensive, commercially-available chemicals into high-value, pharmaceutically-relevant compounds.
The research team's initial work has already led to discovery of what could be the most potent and selective modulator of the estrogen receptor beta, a nuclear hormone receptor that is of great interest in industry as a therapeutic target for a wide variety of illnesses.
"This is an important first step toward establishing a new technology platform to greatly facilitate drug discovery across a diverse landscape of therapeutic indications," said Micalizio.
To demonstrate the value of the technique, the study describes the discovery of a molecule that is selectively toxic to glioblastoma—an aggressive and deadly brain tumor—while showing little effect on non-cancerous human neural stem cells and human astrocytes.
"Glioblastomas are incurable, and existing therapies have horrific side effects," said Arti Gaur, an assistant professor of neurology at Dartmouth's Geisel School of Medicine. "It is extremely exciting and encouraging to see that these novel compounds can selectively kill patient-derived brain tumor cells without harming cells from normal, healthy brain tissue."
The team is currently conducting in vivo testing of the new therapeutic agent and further developing chemical aspects of the emerging technology platform.
The work is part of an effort to explore and demonstrate the power of advances in organic chemistry to enable discovery of therapeutics for a wide range of indications, including neurodegeneration, neuroinflammation, and a wide variety of malignancies.
More information: Nature Communications (2019). DOI: 10.1038/s41467-019-10415-6
Journal information: Nature Communications
Provided by Dartmouth College