Solvent-free, catalyst-free way to produce alkali metal hydrides

Researchers at the U.S. Department of Energy's Ames Laboratory have found a way to create alkali metal hydrides without the use of solvents or catalysts. The process, using room temperature mechanical ball milling, provides a lower cost method to produce these alkali metals which are widely used in industrial processes as reducing and drying agents, precursors in synthesis of complex metal hydrides, hydrogen storage materials, and in nuclear engineering."
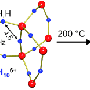
"These alkali metals – lithium, sodium, potassium, rubidium and cesium – form hydrides that are relatively stable, salt-like compounds," said Vitalij Pecharsky, Ames Laboratory scientist and Iowa State University Anson Marston Distinguished Professor of Materials Science. "Typically, you need high temperature (for example, 700° C for lithium) to melt them but even then, a hydride crust forms on the liquid which prevents additional hydride molecules from forming, so it also requires a catalyst."
The process discovered at Ames Laboratory uses mechanical ball milling in the presence of hydrogen gas and is performed at room temperature. Ball milling continuously fractures the alkali metal particles, exposing "clean" surfaces to the hydrogen. This promotes the solid-gas reaction, so the metal hydrides form quite easily without the need for a catalyst.
"One problem with ball-milling is that lithium and related metals are ductile, so they have a tendency to clump up or turn gummy when you mill them," Pecharsky said. "But we found that by simply adding a small amount of lithium-hydride (LiH) or monohydride of the corresponding alkali metals early in the process, it eliminates cold welding."
Besides eliminating the cost of having to heat the metal and adding a catalyst, the room-temperature ball milling also results in impurity-free alkali-metal hydride end products.
"Lithium in particular is reactive when heated to its melting point," Pecharsky said. "It can react with the crucible which introduces impurities."
While increasing the pressure of the hydrogen gas accelerated the hydrogenation process, the research found that the process will work with high-capacity industrial milling operations with minimal modifications.
The research, Solvent- and catalyst-free mechanochemical synthesis of alkali metal monohydrides, was published in the July issue of the Journal of Materials Chemistry A, 2016, 4, 12188. The work was supported by DOE's Office of Science.
More information: Ihor Z. Hlova et al. Solvent- and catalyst-free mechanochemical synthesis of alkali metal monohydrides, J. Mater. Chem. A (2016). DOI: 10.1039/C6TA04391G
Journal information: Journal of Materials Chemistry A
Provided by US Department of Energy