Going against the grain—nitrogen turns out to be hypersociable

Nitrogen is everywhere—there is four times as much of it as oxygen in Earth's atmosphere. However, it is reluctant to form chemical bonds, especially with more than four atoms. Chemists from Warsaw predict, however, that contrary to the rules of typical chemistry, in appropriately selected conditions, there may be a nitrogen that nobody has ever seen—one capable of forming up to six chemical bonds.
Nobody expected this. Computer simulations suggest that nitrogen, with its reluctance to react, could, at a high enough pressure, break the chemical rules and become extremely gregarious—a single atom would then be able to form even six chemical bonds. This surprising discovery has been made by researchers at the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw and the New Technology Centre at the University of Warsaw (CeNT UW).
Chemists have long known that nitrogen may occasionally have a valency of five, which means that it is potentially able to form bonds with five other atoms. Another element with similar properties is phosphorus, which is adjacent to nitrogen in the periodic table.
"Nitrogen, however, behaves differently to phosphorus," says Dr. Patryk Zaleski-Ejgierd (IPC PAS). "In practice, it forms five bonds with at most four other atoms, and more usually with three, as in the popular nitric acid HNO3. We decided to investigate in silico the possibility of obtaining a compound in which pentavalent nitrogen would combine with five neighbours by covalent interactions—chemical bonds. We analyzed thousands of crystal structures of nitrogen compounds with fluorine arising at high pressures. We were hoping to see some structures containing nitrogen pentafluoride NF5 particles. We were completely unprepared for the fact that in one of the crystals, we ran into ions with the formula NF6- in which the nitrogen atom bonds with as many as six fluorine atoms."
Dr Dominik Kurzydlowski (CeNT UW), co-author of the publication in the journal Scientific Reports, explains the essence of the discovery: "Two electrons are typically required for the formation of a single covalent bond. The problem with nitrogen is that when creating various compounds, it 'trades' electrons so as to be constantly surrounded by eight of them. This constrains the total number of atoms bonded to nitrogen to no more than four. We were the first to find a stable crystal in which nitrogen breaks the octet rule, i.e. the requirement to possess exactly eight electrons. It then forms bonds involving a total of up to 12 electrons."
Compounds in which an element breaks the octet rule are called hypervalent. Many elements can form hypervalent compounds, including phosphorus, sulphur and various metals. This phenomenon is advantageous because it significantly widens the number of possible compounds the element can form. For example, if it were not for hypervalency, sulphur would not form sulphuric acid and phosphorus could not be one of the building blocks of DNA.
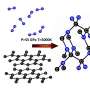
The calculations and simulations associated with the search for hypervalent nitrogen were carried out at the IPC PAS using density functional theory—that is, the method normally used in solid-state calculations. However, the discovery's authors used one of the more advanced embodiments of this theory, the hybrid functional. It enables highly accurate description of chemical bonds, but it takes much longer to perform the calculations.
"The compounds we tested, as well as the conditions under which these compounds were formed, were very exotic. The accuracy of the calculations was therefore our absolute priority which is why we decided to use the hybrid functional for the calculations," says Dr. Kurzydlowski, and stresses that carrying out the simulation within a reasonable timeframe was possible thanks to cooperation with the Interdisciplinary Centre for Mathematical and Computational Modelling, University of Warsaw.
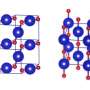
A thorough analysis of the results of computer simulation allowed the researchers to identify the unique crystal structure that, with an increase in pressure, automatically ionizes in a very particular way. A reorganization takes place during which the molecular crystal, originally formed of a mixture of gases NF3 and F2, transforms into a complex ionic crystal constructed of NF4-, NF2+ and... NF6- ions.
The pressure required for the synthesis of crystals containing NF6- amounts to 400 to 500 thousand atmospheres, which is within the reach of current experimental techniques. Simulations suggest that after formation, the crystals would remain metastable, even at much lower pressures. Does that also mean under normal atmospheric pressure?
"I wouldn't bet too much money on that, but it cannot be completely ruled out that one day you will simply be able to pick up the crystals we predict with unique NF6- ions. If they do turn out to be so stable, who knows? Perhaps it will be possible to find some interesting applications for them," says Dr. Zaleski-Ejgierd.
However, taking a crystal with NF6- ions into your hand would probably not be a very good idea. Nitrogen trifluoride is already a strong oxidizing agent that must be stored in steel cylinders. A crystal of nitrogen pentafluoride mixed with NF6- would be an even stronger oxidant, and we can assume that the construction of a container allowing for its safe storage could cause engineers considerable difficulties.
More information: Dominik Kurzydłowski et al, Hexacoordinated nitrogen(V) stabilized by high pressure, Scientific Reports (2016). DOI: 10.1038/srep36049
Journal information: Scientific Reports
Provided by Polish Academy of Sciences