Enhancement in rate of photocatalysis upon catalyst recycling
In chemistry, in order to achieve efficiency of any chemical reaction, a catalyst is added that serves to ideally increase the rate of reaction without any deleterious effects.
Specifically, a photocatalyst is one which is activated under light and can lead to improvement in rates of a variety of redox based reactions. An aspect of photocatalysis that normally does not get much recognition is recyclability.
In fact, for a catalyst to be viable it must be able to withstand the reaction conditions repeatedly and the rates of reaction, upon re-using the catalysts, must not change dramatically (or at all infact).
In reality, however, most catalysts eventually undergo degradation.
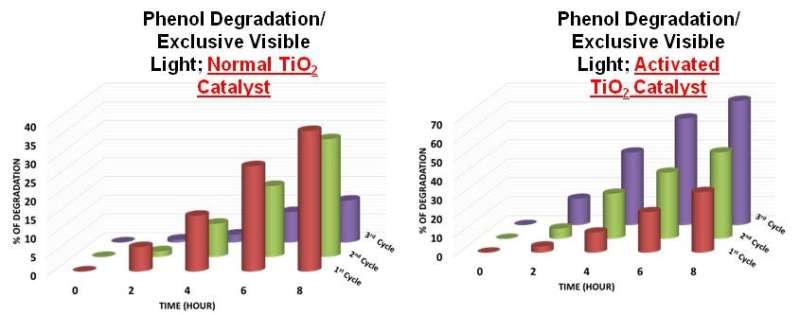
Presented in this work from Deepa Khushalani's group is a seminal observation where they noted an increase in rate of reaction upon recycling. The increase was 1.7 times higher in the second cycle (when compared to the first) and it was 3.1 times higher in the third cycle (again, when compared to the first).
Moreover we have shown that photocatalysts can in fact undergo structural evolution and, in certain conditions, evolve to be a more effective additive for subsequent reusability.
This aspect is unique since the understanding, previous to this work, was that catalysts remain unchanged during the reactions.
More information: Kalpesh Sorathiya et al, Enhancement in Rate of Photocatalysis Upon Catalyst Recycling, Scientific Reports (2016). DOI: 10.1038/srep35075
Journal information: Scientific Reports
Provided by Tata Institute of Fundamental Research