Digital microbes for munching yourself healthy
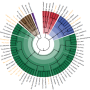
Hundreds of bacterial species live in the human gut, helping to digest food. The metabolic processes of these bacteria are not only tremendously important to human health – they are also tremendously complex. A research team at the Luxembourg Centre for Systems Biomedicine (LCSB) of the University of Luxembourg has taken an important step in modelling the complexity of the human gut's bacterial communities – the microbiome – on the computer. The researchers gathered all known data on the metabolism of 773 bacterial strains – more than ever before. Working from this data, they developed a computer model for each bacterial strain. This collection, known as AGORA, can now be used on the computer to simulate metabolic processes taking place in the microbes and to investigate how they affect the metabolism of other microbes and that of the human host. The LCSB team publishes its results in the scientific journal Nature Biotechnology. The collection of predictive metabolic models is available to researchers via vmh.life.
The bacterial species living in the human gut not only help to digest food, but also produce valuable vitamins and even affect the way we metabolise drugs. The metabolic processes of these bacteria are crucial to health, and are highly complex: The bacteria are in constant contact with gut cells, and the different organisms continually influence one another. Thus, they play as important a role in health as they do in numerous diseases. Despite many advances in science, knowledge of these microbes is still limited. To improve understanding and to aid novel discoveries, the research team led by LCSB scientist Prof. Dr. Ines Thiele, head of the "Molecular Systems Physiology" group, has now created the most comprehensive collection of computational models for 773 different gut microbes, capturing their individual metabolisms, called AGORA. "AGORA is based on a new concept for the comparative reconstruction of bacterial metabolic models," says Ines Thiele: "It allows the analysis of a much greater number of bacterial strains than was ever possible before. With AGORA, and by including other datasets, we can systematically study the metabolic interactions within the gut microbiome and how these interactions are influenced by external factors, including the diet and host metabolism."
The first author of the study, Stefania Magnusdottir, is currently doing her PhD degree in Ines Thiele's group at the LCSB: "The basis for our paper was a thorough investigation of the literature on microbial metabolism," she explains. "We gathered known experimental and genomic data on the metabolism of 773 bacterial strains to refine and validate the computational models. Based on this, we characterised each microbe's metabolism and found that both their metabolic capabilities and our diet play important roles in how the microbes interact with each other. We can generate personalised microbiome models by integrating these computational models with metagenomic data, which can be obtained by sequencing the microbes present in stool samples of healthy and sick individuals."
"With our models, we can search, in a targeted manner, for metabolic pathways that are fundamentally important to the microbiome in the gut, and we can work out what could trigger diseases when these metabolic processes go wrong," says co-author Dr. Ronan Fleming, who leads the Systems Biochemistry group at the LCSB: "The AGORA models will now allow us to study the impact of host-microbiome interactions in specific diseases or to use them in the emerging field of personalised medicine."
Using AGORA to study the gut microbiome will involve close collaboration with researchers who are investigating the gut microbiome in the laboratory, including Prof. Dr Paul Wilmes, head of the LCSB Eco-Systems Biology group. His group has developed methods for studying gut bacteria under real-life conditions. "AGORA directs us to targeted bacterial metabolic processes to perform focused experiments, allowing precise and comprehensive modelling of processes within the gut microbes," Paul Wilmes asserts.
For Ines Thiele, the high degree of precision is not an end in itself: "We want to understand how the microbes modulate human metabolism when we modify our diet. This may give us clues as to how we may prevent, or even treat, diseases, for example by identifying dietary supplements that could modify the interactions within a diseased gut microbiome to imitate the metabolic functions of a healthy one."
More information: Stefanía Magnúsdóttir et al. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota, Nature Biotechnology (2016). DOI: 10.1038/nbt.3703
Journal information: Nature Biotechnology
Provided by University of Luxembourg