Drug manufacturers need to understand how their products affect entire biological networks
Decoys in DNA may serve a larger purpose than drug designers suspect, according to Rice University scientists.
These decoy sequences bind the same proteins that prompt gene expression elsewhere along DNA. But they may have greater function in cells than simply keeping proteins out of circulation, according to researchers at Rice's Center for Theoretical Biological Physics.
The takeaway, said Rice biophysicist Peter Wolynes, is that drug manufacturers need to understand how their products affect entire biological networks, not just how they bind to their intended molecular targets.
Wolynes and associates Zhipeng Wang and Davit Potoyan build computer simulations of such networks to understand how they tick. Wang and Potoyan are lead authors of a paper about this research in the Royal Society journal Interface.
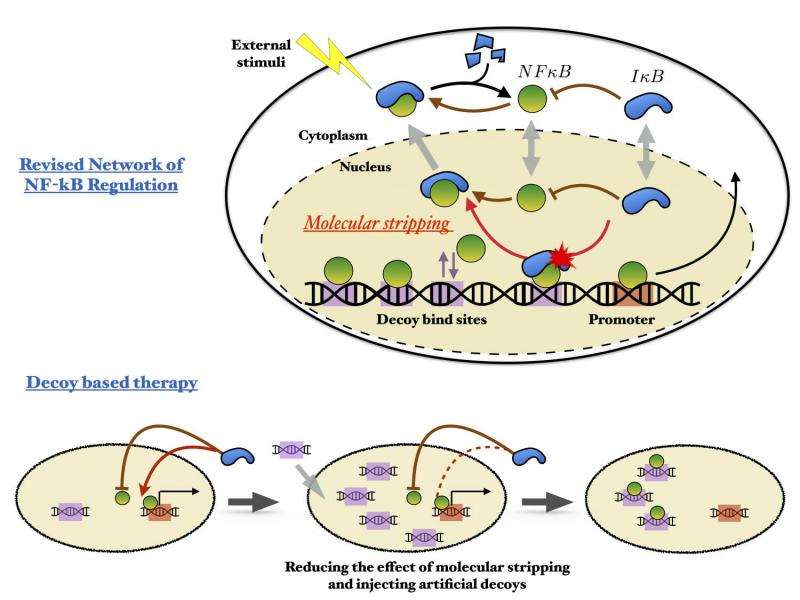
The trio's ongoing work led them to take a deep look at the master regulatory circuit in cells that involves transcription proteins called NFkB. These proteins work along with DNA and a family of protein inhibitors, IkB, to orchestrate cell death, immune response to inflammation and the proliferation and differentiation of cells. Misregulation of NFkB has been implicated in cancer, arthritis, chronic inflammation, asthma, neurodegenerative diseases and heart disease.
In normal operation, NFkB binds to DNA and prompts the expression of IkBa. But some of the NFkB molecules bind instead to decoy sequences, not their functional targets. There they all sit until IkBa molecules eventually trigger a recently discovered stripping process that releases all the NFkB at once and quenches transcription. The oscillating pulses of NFkB regulate the expression of many proteins, but the role of the nonfunctional decoys has been a mystery.
"Some transcription factors can bind to many sites, and sometimes this binding is nonspecific, with no apparent purpose," said Potoyan, a postdoctoral researcher in Wolynes' lab.
"Traditionally, people look for things that change dramatically," he said. "They look for something that turns on, something synthesizing, something degrading. Decoy binding doesn't appear to be very interesting, but we're saying it can influence system-level dynamics in a quiet way by sequestering transcription factors from being active in the nucleus.
"In this case, we illustrate the decoy problem with the NFkB example showing how decoys can change the frequency and modulate the amplitude of the circuit."
Wolynes said IkB strips proteins both from the active and the decoy DNA without discretion. "One consequence is that it becomes harder to recognize an active site from a decoy, throwing a considerable monkey wrench into the bioinformatics problem of how to find interesting parts of the genome."
He noted there have been attempts to treat NFkB misregulation by adding artificial oligonucleotide decoys. "But I don't think these treatments are yet as successful as they could be," Wolynes said. "This paper shows the effect of the decoys will be diminished by stripping. One of the 'translational' suggestions in this paper is you've also got to control the level of IkB to use decoys more effectively, and that suggests some kind of combination therapy."
Wang, a Rice graduate student, hopes the team's increasingly comprehensive models of gene networks will help drug designers. "I want to use physical chemistry and physics principles to provide quantitative guidance to translational researchers and physicians so they can better understand how to cure disease," he said.
More information: Zhipeng Wang et al. Molecular stripping, targets and decoys as modulators of oscillations in the NF-κB/IκBα/DNA genetic network, Journal of The Royal Society Interface (2016). DOI: 10.1098/rsif.2016.0606
Journal information: Journal of the Royal Society Interface
Provided by Rice University