September 14, 2016 report
New CCCCC pentadentate chelates with properties for use in photothermal therapy
(Phys.org)—Macrocyclic and chelating molecules are important in physiological processes and pharmaceuticals. Hemoglobin, for example, is a chelating molecule in which iron is coordinated to donor atoms in a porphyrin ring. Often the donor atoms are some combination of nitrogen, oxygen, phosphorous, and sulfur. For example, nitrogen serves as the donor atoms in hemoglobin. Sometimes carbon acts as a donor atom, but it is usually as a heterocycle with one of these other atoms.
In a study recently published in Science Advances, several researchers from Xiamen University in China report the synthesis and characterization of a pentadentate, five-carbon, chelating molecule. This planar molecule coordinates osmium and is amenable to several substituents. Furthermore, its properties make it a good candidate for photothermal therapy.
The current study builds on work by this group in which they found that the ligand's Möbius aromaticity plays a key role in stabilizing the metal center. Möbius aromaticity stems from molecular orbital theory in which an aromatic ring's pi-donating atomic orbitals rotate around the ring like a Mobius strip. This study reports the synthesis of a ligand with planar 12-center 12-electron dπ-pπ pi-conjugation, the largest planar Möbius aromatic system reported. It is comprised of two fused five-member rings, a six-member ring, and a three-member, non-aromatic ring.
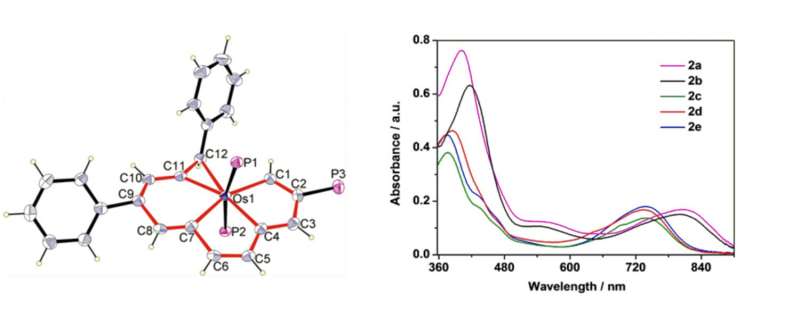
Structurally, the five carbon donor atoms are in the same plane (equatorial) while the two phosphorous atoms are in the axial plane. The osmium metal is almost co-planar with the carbon atoms. The C-Os bond lengths indicate delocalized bonds. As reported by the authors of the study, this structure represents the highest coordination number for a metal atom coordinated to carbon donor atoms in planar geometry.
The synthesis of these planar pentadentate compounds was fairly straight-forward involving a ring expansion with alkynes or allenes with various substituents. The first product, resulting in phenyl substituents on the six-membered ring and the three-member ring was made with 3-ethynylthiophene and silver borohydride at room temperature. This product, as well as the ones with cyclohexane, cyclopropane, and thiophene substituents was characterized with NMR, high-resolution mass spectrometry, and elemental analysis, all of which confirmed a pentagonal bipyramidal structure and delocalized Os-C bonds.
UV-Vis studies and near-IR showed that the absorption spectra changed based on different substituent groups (See figure above). This indicates that the pentadentate compound's photophysical properties change based on structural differences. This could have implications for tuning these compounds for practical uses.
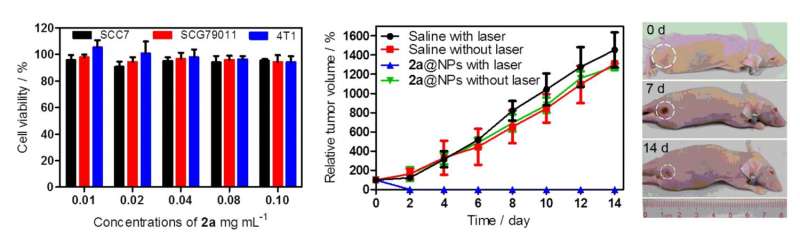
Finally, Zhu, et al. tested whether theses pentadentate compounds could be used for photothermal therapy. For PTT, often IR or near-IR is used to apply heat to cancer cells in an effort to destroy the cells. The various pentadentate compounds were first tested to determine the relationship between the concentration of the pentadentate compound and solution temperature. The compound with benzene substituents showed the most promising temperature changes.
In order to get this pentadentate compound into the body, Zhu, et al. loaded the pentadentate on PEG micelle nanoparticles. They found that when interacted with SCC7 cells, a squamous cell carcinoma line, the tumor volume and cell viability decreased substantially compared to controls. Since in vitro testing worked so well, Zhu, et al. then wanted to see how the pentadentate nanoparticles worked in vivo.
Studies with SCC7 tumor-bearing mice showed that after fourteen days, the control group had tumors that were larger (up to twelve times larger) than at the beginning of the experiment. The group with the nanoparticles, however, showed a significant decrease in tumor size and comparable efficacy to nanotubes and nanoporphyrins.
This work demonstrates a new CCCCC pentadentate structure in which the five-carbon coordination center is co-planar with the metal. These new compounds provide an example of a rare type of aromaticity and have properties that make them excellent candidates for photothermal therapy.
More information: C. Zhu et al. CCCCC pentadentate chelates with planar Mo bius aromaticity and unique properties, Science Advances (2016). DOI: 10.1126/sciadv.1601031
Abstract
The coordinating atoms in polydentate chelates are primarily heteroatoms. We present the first examples of pentadentate chelates with all binding atoms of the chelating agent being carbon atoms, denoted as CCCCC chelates. Having up to five metal-carbon bonds in the equatorial plane has not been previously observed in transition metal chemistry. Density functional theory calculations showed that the planar metallacycle has extended Craig-Möbius aromaticity arising from 12-center–12-electron dπ-pπ π-conjugation. These planar chelates have broad absorption in the ultraviolet-visible–near-infrared region and, thus, notable photothermal performance upon irradiation by an 808-nm laser, indicating that these chelates have potential applications in photothermal therapy. The combination of facile synthesis, high stability, and broad absorption of these complexes could make the polydentate carbon chain a novel building block in coordination chemistry.
Journal information: Science Advances
© 2016 Phys.org