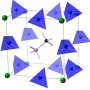
Variations in size, shape and electronic properties of ring-shaped molecules lead to changes in ion-selectivity
Selective ion transport is the foundation of water purification technology, as well as underpinning a range of biological effects—such as the function of nerves—and diagnostic technologies that use ion-sensitive electrodes to detect abnormalities in biological fluids. Now A*STAR researchers have invented a new synthetic ion recognition system and found a way to fine-tune the selectivity of the system that will benefit many applications.
The ability to pick out one type among many metal ions is vital in many fields. Ring-shaped molecules—known as macrocycles—have long been used as synthetic ion recognition systems. The ion binding properties of the macrocycles are defined by the size and shape of their internal cavity and how well this matches with the desired metal ion.
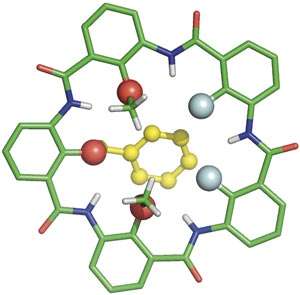
Now, Huaqiang Zeng from the A*STAR Institute of Bioengineering and Nanotechnology, and colleagues from China, have designed a series of macrocycles assembled from five building blocks.
"Over the past eight years, we had studied the properties of pentamers of several different single building blocks," explains Zeng. "Hydrogen bonding between the units means that the macrocycles are formed with very little strain and flexibility, and this in turn means that we can exchange building blocks within the same framework to tune their ion binding properties."
Each of the five building blocks is decorated with a chemical group—fluorine, hydroxy and alkoxy groups—which are projected into the central cavity of the macrocycle. The subtle changes in the steric and electronic properties of these groups define the selectivity of ion binding.
A blending of approaches to constructing new macrocycles can produce a very large library of different macrocycles from relatively few different building blocks. For example, two different building blocks can be assembled in eight different ways, but five building blocks can potentially lead to 629 possible macrocycles.
Zeng and co-workers prepared a small selection of these possibilities and compared their ability to selectively extract specific metal ions from a mixture of 20.
The team was able to identify macrocycles that were selective for the extraction of caesium, silver and potassium ions. "We plan to expand both the set of building blocks and the number of macrocycles," explains Zeng. "This will be the key to identifying macrocycles with higher selectivity and affinity.
Zeng says that, in the longer term, the team plans to use their new ion-binders to construct artificial ion channels for wider applications in diverse fields, including molecular sensing and water purification.
More information: Ying Liu et al. Intramolecularly Hydrogen-Bonded Aromatic Pentamers as Modularly Tunable Macrocyclic Receptors for Selective Recognition of Metal Ions, Journal of the American Chemical Society (2015). DOI: 10.1021/jacs.5b07123
Journal information: Journal of the American Chemical Society