Researchers develop a small-molecule switch to activate proteins
From growing teeth, bones, and tissue in skin and organs to creating enzymes and hormones, proteins are one of the most diverse and important elements of living organisms. But those varied purposes, along with the interconnectivity of all systems in living cells, make it challenging for researchers to determine how they operate.
But, recently, researchers led by University of Pittsburgh Chemistry Professor Alexander Deiters developed a technology that allows a small-molecule phosphine to act as an "off-to-on switch" to control protein activity, giving scientists more control over studies involving the molecular details of biological processes.
"Being able to precisely control specific protein function in cells using a small, drug-like molecule as an external trigger reveals activities related to the protein in isolation and provides the kinetics of cellular processes," said Deiters. Deiters said that their research could lead to applications in gene therapy and be used as a research tool to better understand disease processes. "Similar to turning on a light switch to see who is grabbing a late-night snack in the kitchen, a switch that rapidly activates proteins allows us to learn more about their behavior and function."
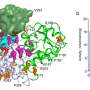
Proteins are composed of long chains of amino acids, and, in their study, the researchers found that adding an unnatural amino acid, called ortho-azidobenzyloxycarbonyl lysine, to a specific site in proteins "protected" them, or rendered them inactive. When the researchers treated cells expressing the protected protein with a phosphine reagent, the unnatural amino acid was converted back to natural lysine, "deprotecting" it and forming an active, wild-type protein.
Having a triggered on-switch enables researchers to observe the protein's activity in isolation. It also helps to separate it from its interaction with other parts of the cell, leading to a better understanding of the protein's role as well as its relationship and interaction with other components of the cell.
In their study, the researchers used the small molecule switch in four cellular processes:
- bioluminescence—by deprotecting the enzyme luciferase, the researchers were able to trigger cells to emit light;
- fluorescence—by activating a protein originally found in jellyfish, the researchers were able to convert blue light into green light;
- protein translocation—the researchers were able to use the small molecule switch to assist in moving proteins between different cellular compartments; and
- gene editing and DNA recombination—the switch enabled researchers to control the inserting and removing of genetic information.
More information: Ji Luo et al. Small-molecule control of protein function through Staudinger reduction, Nature Chemistry (2016). DOI: 10.1038/nchem.2573
Journal information: Nature Chemistry
Provided by University of Pittsburgh