How cancer cells spread and squeeze through tiny blood vessels
The spread of cancer from a tumor's original location to other parts of the body can play a major role in whether the disease turns deadly. Many steps in this process, called metastasis, remain murky. But now scientists are gaining new insights into how cancer cells might squeeze through and even divide within narrow blood vessels while travelling in the body. They report their study using microtubular nanomembranes in the journal ACS Nano.
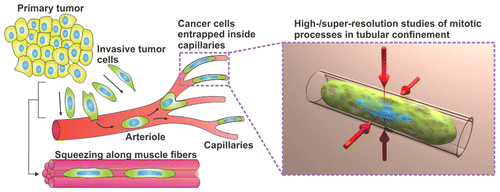
One thing scientists do know about metastasis is that spreading cancer cells elongate to fit through capillaries—blood vessels as fine as spider silk. They can get trapped in these skinny passages, but despite becoming misshapen, they seem to still be able to divide and form little colonies of cells before dislodging and moving on. If scientists could better understand this process, they could potentially improve anti-metastatic treatment strategies. But studying it in molecular detail is not possible with conventional analytical techniques. So Wang Xi, Christine K. Schmidt and colleagues used transparent, rolled-up nanofilms to study how cancer cells divide in capillaries.
The researchers trapped live cancer cells in the tubular membranes and, with optical high- and super-resolution microscopy, could see how the cells adapted to the confined environment. Cell structures significantly changed in the nanomembranes, but it appeared that membrane blebbing—the formation of bulges—at the cells' tips helped keep genetic material stable, an important requirement for healthy cell division. The researchers say their technique could be a useful tool for further investigating metastatic cancer.
More information: Wang Xi et al. Molecular Insights into Division of Single Human Cancer Cells in On-Chip Transparent Microtubes, ACS Nano (2016). DOI: 10.1021/acsnano.6b00461
Abstract
In vivo, mammalian cells proliferate within 3D environments consisting of numerous microcavities and channels, which contain a variety of chemical and physical cues. External environments often differ between normal and pathological states, such as the unique spatial constraints that metastasizing cancer cells experience as they circulate the vasculature through arterioles and narrow capillaries, where they can divide and acquire elongated cylindrical shapes. While metastatic tumors cause most cancer deaths, factors impacting early cancer cell proliferation inside the vasculature and those that can promote the formation of secondary tumors remain largely unknown. Prior studies investigating confined mitosis have mainly used 2D cell culture systems. Here, we mimic aspects of metastasizing tumor cells dividing inside blood capillaries by investigating single-cell divisions of living human cancer cells, trapped inside 3D rolled-up, transparent nanomembranes. We assess the molecular effects of tubular confinement on key mitotic features, using optical high- and super-resolution microscopy. Our experiments show that tubular confinement affects the morphology and dynamics of the mitotic spindle, chromosome arrangements, and the organization of the cell cortex. Moreover, we reveal that membrane blebbing and/or associated processes act as a potential genome-safety mechanism, limiting the extent of genomic instability caused by mitosis in confined circumstances, especially in tubular 3D microenvironments. Collectively, our study demonstrates the potential of rolled-up nanomembranes for gaining molecular insights into key cellular events occurring in tubular 3D microenvironments in vivo.
Journal information: ACS Nano
Provided by American Chemical Society