The use of Camelid antibodies for structural biology
The use of Camelid antibodies has important implications for future development of reagents for diagnosis and therapeutics in diseases involving a group of enzymes called serine proteases.
Antibodies have many applications, in areas ranging from basic molecular biology and biochemistry to clinical medicine. Recently, much interest has been focused on a special group of antibodies from Camelids (camels, dromedaries, llamas). Due to their small size and unique structures, these Camelid antibodies are ideal building blocks for the generation of novel biological drugs, with multiple competitive advantages over other therapeutic molecules. Moreover, Camelid antibodies offer many technical applications in basic biochemistry and structural biology.
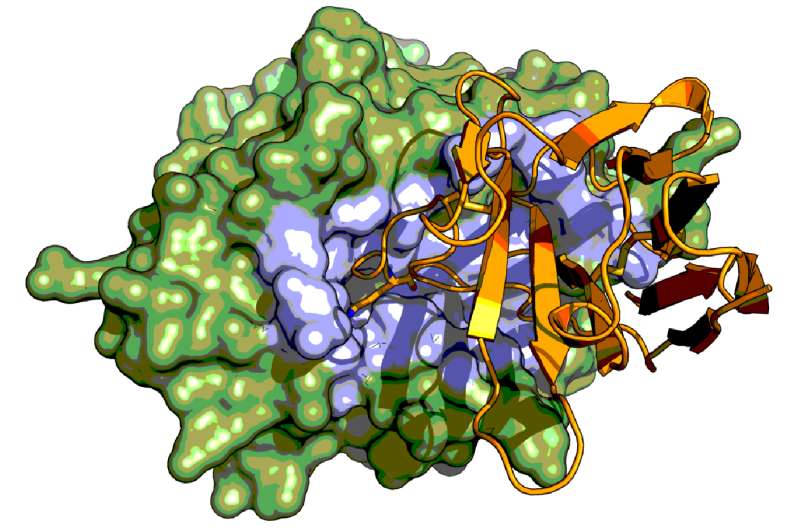
Now, a group of Danish, Belgian, and Chinese researchers have used the Camelid antibody technology to explore basic principles of catalysis and inhibition of a group of enzymes called serine proteases, using X-ray crystal structure analysis. Serine proteases catalyse important processes in blood coagulation, fibrinolysis, tissue remodeling, and other physiological functions.
The research team has developed Camelid antibodies against serine proteases, thereby allowing a structural characterization of a long standing problem concerning whether a certain peptide segment will behave as an inhibitor or a substrate for a serine protease. The results also have important implications for future development of reagents for diagnosis and therapeutics in diseases involving serine proteases.
The results have been obtained in a collaboration between researchers from Aarhus (Denmark), Leuven and Brussels (Belgium) and Hong Kong (China). In particular, Serge Muyldermans, Vrijes Universiteit Brussels, who originally pioneered the development of the Camelid antibody technology, has contributed with his expertise. Tobias-Kromann-Hansen, as part of his Ph.D.-study, spent four months in his laboratory. Tobias Kromann-Hansen is currently developing these studies further as a Carlsberg Postdoctoral Research Fellow at the University of California San Diego, USA. The results are being published in the Journal of Biological Chemistry. The publication is one of the achievements of the Danish-Chinese Centre for Proteases and Cancer, headed by Peter A. Andreasen.
More information: Tobias Kromann-Hansen et al. A Camelid-derived Antibody Fragment Targeting the Active Site of a Serine Protease Balances between Inhibitor and Substrate Behavior, Journal of Biological Chemistry (2016). DOI: 10.1074/jbc.M116.732503
Journal information: Journal of Biological Chemistry
Provided by Aarhus University