Ultrafast pulses shed light on photochemical processes
Tunnelling ionization studies by researchers in Japan and Russia show changes in electron distributions between ground- and excited-state in laser tunnelling ionization of molecules.
The change in electronic distributions in molecules as they are photoexcited can offer useful insights for photochemistry. Studying the position and momentum of molecular fragments after ionization by "tunnelling" in an intense laser field can provide a means of mapping these electron distributions, but excited state molecules can be awkward to probe in this way. Now a collaboration of researchers at Nagoya University, The Open University of Japan, the University of Electro-communications, Tokyo (UEC, Tokyo), and the Moscow Institute of Physics and Technology, has successfully applied the approach to excited states of nitrogen oxide (NO).
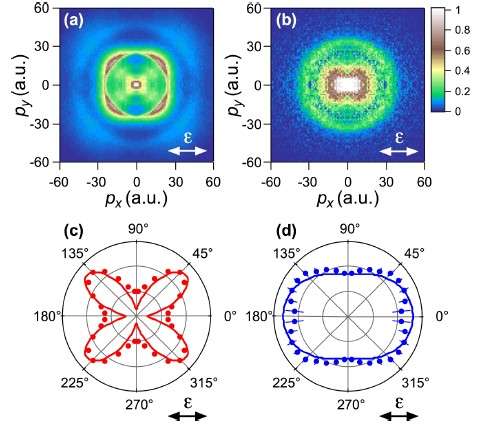
The researchers, including Toru Morishita at UEC, Tokyo, observed a change in the peak of the momentum of N+ fragments depending on the initial state: 45° with respect to the polarization of the laser beam when probed in the ground state and 0° for the excited states. The researchers compared the results with weak-field asymptotic theory, which was established by the Morishita Group, and noted "excellent agreement for both the ground and excited states".
The main difficulty in probing the electron distribution of excited state molecules in this way is the diminished tunnelling potential. As a result multiphoton processes are more likely to contribute to ionization. The researchers probed the mechanism at work by altering the polarization of the laser field. A linearly polarised field sends the electron towards and an electron impact process causes the excited ionized state. For more circularly polarised fields, the electron trajectory shifts away from the nucleus so that fragment yields decrease, as observed.
In their report of the results the researchers conclude, "The present study provides a deeper understanding of laser tunnelling ionization of molecules, a key step of important applications such as high-order harmonics generation and self-electron diffraction, and paves the way for real-time visualization of electron dynamics in chemical reactions."
Background
Highest occupied molecular orbital (HOMO)
The electrons orbiting a nucleus occupy different discrete energy levels. The number of electrons that can occupy each level is limited so that once the lowest level is filled electrons will occupy the level above. The electrons furthest from the nucleus are described as the highest occupied molecular orbital (HOMO) and this orbital is particularly important for photochemistry.
The distribution of the electron cloud orbiting around a nucleus has a different shape for different orbitals, energy levels and occupancies. Excitation of an electron in an atom or a molecule changes the electronic distribution.
The electronic configuration of NO in the ground state is 1σ22σ23σ24σ25σ21π42π13sσ0, where the Greek symbols denote sigma and pi electron orbitals, the numbers preceding them the energy level and the superscripts the occupancy. When NO is photoexcited, an electron moves from 2π to 3sσ to give the configuration 1σ22σ23σ24σ25σ21π42π03sσ1.
Tunnelling ionization
Tunnelling is a quantum mechanical process that allows a system to overcome/penetrate an energy barrier. When exposed to an intense polarised laser field, a molecule can ionize through tunnelling and the HOMO electronic distribution with respect to the polarisation of the laser reflects the likelihood of ionization. This has been used to study spatially aligned or oriented molecules such as N2, O2, CO2 and OCS.
In the current study the researchers used the position and momentum of expelled ionized fragments with respect to the laser polarisation to map the electronic distribution for the randomly aligned NO molecule. The ionization energy for NO from the excited state is 3.8 eV, far lower than the energy peaks measured in the study. Tunnelling ionization processes cause the molecule to dissociate into N+ and O.
The timescale of the dissociation in ionization is much shorter than the rotational periods of NO so the plots can be understood to represent the molecular-axis distributions after ionization.
More information: Tomoyuki Endo et al. Imaging Electronic Excitation of NO by Ultrafast Laser Tunneling Ionization, Physical Review Letters (2016). DOI: 10.1103/PhysRevLett.116.163002
Journal information: Physical Review Letters
Provided by University of Electro-Communications